Do Transition Metals Have A Charge
Muz Play
Mar 25, 2025 · 6 min read
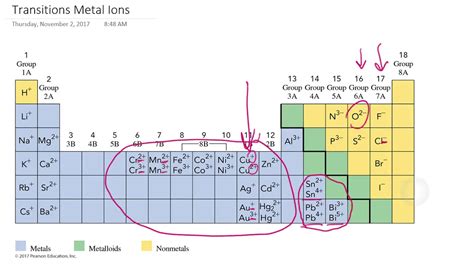
Table of Contents
Do Transition Metals Have a Charge? Understanding Oxidation States and Variable Valency
Transition metals are renowned for their fascinating and complex chemistry, largely driven by their ability to exhibit multiple oxidation states. This characteristic directly relates to the question: do transition metals have a charge? The answer is nuanced, requiring a deeper understanding of their electronic configuration and bonding behavior. While they don't possess a fixed, inherent charge like simple ions, they readily form ions and participate in chemical reactions with varying charges, often referred to as oxidation states. This article will delve into the intricacies of transition metal charges, exploring their electronic structure, the factors influencing variable valency, and the implications for their diverse chemical properties.
The Electronic Structure: A Foundation for Variable Charges
Understanding why transition metals exhibit variable charges begins with their electronic structure. Unlike alkali metals or alkaline earth metals which readily lose one or two electrons to achieve a stable noble gas configuration, transition metals have partially filled d-orbitals. These d-orbitals are relatively close in energy to the s-orbitals in the outermost shell. This proximity allows for electrons to be readily lost from both s and d orbitals during the formation of ions, resulting in a range of possible oxidation states.
For example, consider iron (Fe). Its electronic configuration is [Ar] 3d⁶ 4s². Iron can lose two electrons from the 4s orbital to form the Fe²⁺ ion (ferrous ion), or it can lose three electrons (two from 4s and one from 3d) to form the Fe³⁺ ion (ferric ion). This ability to lose varying numbers of electrons gives rise to the variable oxidation states of iron, and indeed, many other transition metals.
The Role of d-Orbitals in Variable Oxidation States
The partially filled d-orbitals are central to the variable charge exhibited by transition metals. The energy difference between the d-orbitals is relatively small, meaning that electrons can be easily excited to higher energy levels or removed altogether, depending on the chemical environment. This contrasts sharply with main group elements, where the energy difference between orbitals is larger, leading to a more predictable and limited range of oxidation states.
The ability of transition metals to form complex ions further enhances the complexity of their charge behaviour. Ligands, which are molecules or ions that bond to the central metal ion, can affect the energy levels of the d-orbitals through ligand field effects. These effects can stabilize certain oxidation states over others, influencing the overall charge observed in a particular compound.
Factors Influencing Variable Valency
Several factors contribute to the variable valency observed in transition metals:
1. Ligand Field Stabilization Energy (LFSE)
The interaction between the metal ion and its surrounding ligands significantly affects the energy of the d-orbitals. This ligand field stabilization energy (LFSE) can favor certain oxidation states over others. For example, strong-field ligands can lead to a large splitting of the d-orbitals, making certain oxidation states more stable than others. The geometry of the complex also plays a role in determining the LFSE.
2. Electronegativity of Ligands
The electronegativity of ligands influences the electron density around the metal ion. Highly electronegative ligands can withdraw electron density from the metal, making higher oxidation states more favorable. Conversely, less electronegative ligands may stabilize lower oxidation states.
3. Size and Charge of the Metal Ion
The size and charge of the metal ion itself affect its ability to accommodate different numbers of ligands and electrons. Larger ions tend to have lower charge densities, making higher oxidation states less favorable due to increased electrostatic repulsion.
4. The Nature of the Reaction Conditions
Reaction conditions, such as pH, temperature, and the presence of oxidizing or reducing agents, can influence the oxidation state of the transition metal involved. For instance, a highly oxidizing environment might favor higher oxidation states, while a reducing environment may favor lower oxidation states.
Common Oxidation States of Transition Metals
Transition metals exhibit a wide range of oxidation states. Some common examples include:
- Manganese (Mn): +2, +3, +4, +6, +7
- Iron (Fe): +2, +3
- Chromium (Cr): +2, +3, +6
- Copper (Cu): +1, +2
- Vanadium (V): +2, +3, +4, +5
The most common oxidation state for a given transition metal often depends on the specific chemical environment. The interplay between the factors discussed above determines which oxidation state is most stable under given conditions.
Implications of Variable Charges in Transition Metal Chemistry
The ability of transition metals to exist in multiple oxidation states has profound implications for their chemistry and applications:
1. Catalysis
Transition metals are incredibly important catalysts in many industrial processes. Their variable oxidation states allow them to readily accept and donate electrons, facilitating chemical reactions. Many catalytic cycles involve the transition metal cycling between different oxidation states.
2. Color and Magnetism
The partially filled d-orbitals of transition metals are responsible for their often vibrant colors and magnetic properties. The electronic transitions between different d-orbital energy levels give rise to the absorption of specific wavelengths of light, resulting in the characteristic colors of many transition metal compounds. The presence of unpaired electrons in the d-orbitals also leads to magnetic properties such as paramagnetism or ferromagnetism.
3. Biological Systems
Transition metals play crucial roles in biological systems. For instance, iron is essential for oxygen transport in hemoglobin, while copper is involved in various enzymatic processes. The variable oxidation states of these metals allow them to participate in redox reactions, essential for life.
4. Materials Science
The tunable properties of transition metals make them invaluable in materials science. Their ability to form alloys with varying compositions and oxidation states allows for tailoring the properties of materials for specific applications. This is utilized extensively in the development of new alloys with desired strength, conductivity, and other properties.
Conclusion: A Complex but Crucial Aspect of Transition Metal Chemistry
The question of whether transition metals have a charge is best answered with a qualified "yes, but it's complex." They don't possess a single, fixed charge like alkali metals; instead, they exhibit variable oxidation states depending on a variety of factors, including electronic configuration, ligand field effects, electronegativity of ligands, and reaction conditions. This ability to adopt multiple oxidation states is responsible for the rich and diverse chemistry of transition metals, making them essential in catalysis, materials science, and biology. Understanding these variable charges is crucial for comprehending the behavior and applications of these fascinating elements. Further research continues to expand our knowledge of the intricate interplay of factors governing the oxidation states and overall reactivity of transition metals, revealing ever more nuanced aspects of their complex chemistry. The field remains active and vibrant, with ongoing efforts to synthesize novel compounds and explore new applications of these versatile elements. This dynamic nature underscores the continued importance of studying and understanding the multifaceted world of transition metal chemistry.
Latest Posts
Latest Posts
-
The Cutaneous Membrane Is Also Known As
Mar 26, 2025
-
Solve The Following System Of Equations Algebraically
Mar 26, 2025
-
Why Are Tertiary Carbocations More Stable
Mar 26, 2025
-
How To Draw An Integral Sign
Mar 26, 2025
-
Which Plane Divides The Body Into Superior And Inferior Sections
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Do Transition Metals Have A Charge . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
