An Blan Is Dissolved In A Solvent
Muz Play
Mar 21, 2025 · 6 min read
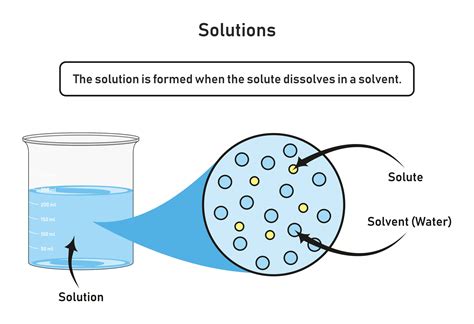
Table of Contents
When a Solute Dissolves in a Solvent: A Deep Dive into the Dissolution Process
Understanding how a solute dissolves in a solvent is fundamental to chemistry and numerous applications across various fields. From pharmaceutical drug delivery to environmental remediation, the principles governing dissolution are crucial. This comprehensive article will explore the intricacies of this process, delving into the factors that influence it and the various theoretical models used to describe it.
The Basics: Solutes, Solvents, and Solutions
Before diving into the mechanics of dissolution, let's define some key terms. A solute is the substance that dissolves in another substance. A solvent is the substance that dissolves the solute. The resulting homogeneous mixture is called a solution. Think of making sweet tea: sugar (the solute) dissolves in water (the solvent) to create a sweet tea solution.
The ability of a solvent to dissolve a solute is termed solubility. Solubility is expressed as the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure. It's crucial to remember that solubility is not a fixed value; it's highly dependent on several factors, as we'll discuss below.
Types of Solutions
Solutions can be categorized based on the state of matter of the solute and solvent. Common types include:
- Solid solutions: A solid solute dissolved in a solid solvent (e.g., alloys like brass).
- Liquid solutions: A solid, liquid, or gaseous solute dissolved in a liquid solvent (e.g., saltwater, sugar in water).
- Gaseous solutions: A gas solute dissolved in a gas solvent (e.g., air).
The Dissolution Process: A Molecular Perspective
At a molecular level, dissolution is a dynamic process involving several steps:
-
Solvation: The solvent molecules surround the solute molecules, weakening the attractive forces holding the solute molecules together. This process is often referred to as solvation. For aqueous solutions (where water is the solvent), this is specifically called hydration.
-
Dissociation: For ionic compounds (like salts), the solvent molecules can overcome the electrostatic forces holding the ions together, causing them to dissociate into individual ions. This leads to the formation of solvated ions, where each ion is surrounded by a shell of solvent molecules.
-
Diffusion: Once the solute particles are separated and surrounded by solvent molecules, they diffuse throughout the solvent, resulting in a homogeneous mixture. This diffusion process is driven by the tendency of the system to achieve maximum entropy (disorder).
The Role of Intermolecular Forces
The success of dissolution heavily depends on the interplay of intermolecular forces between the solute and solvent molecules. Like dissolves like is a fundamental principle:
-
Polar solvents (like water) effectively dissolve polar solutes (like sugars and salts) due to strong dipole-dipole interactions and hydrogen bonding. The polar nature of water allows it to effectively surround and separate the charged or polar solute molecules.
-
Nonpolar solvents (like hexane) effectively dissolve nonpolar solutes (like oils and fats) due to weak London dispersion forces. Nonpolar solvents cannot interact effectively with polar solutes, resulting in low solubility.
For instance, oil (a nonpolar substance) will not dissolve in water (a polar substance) because the intermolecular forces between oil molecules and water molecules are significantly weaker than the forces within each substance.
Factors Affecting Dissolution Rate
The rate at which a solute dissolves in a solvent isn't solely determined by solubility; it's also influenced by several factors:
1. Temperature:
Generally, increasing the temperature increases the dissolution rate. Higher temperatures provide more kinetic energy to the solvent molecules, leading to more frequent and energetic collisions with solute particles, thus accelerating the dissolution process. However, the effect of temperature on solubility can be complex and varies depending on the solute and solvent.
2. Surface Area:
Increasing the surface area of the solute (e.g., by crushing a solid into smaller particles) dramatically increases the dissolution rate. A larger surface area exposes more solute particles to the solvent, leading to more frequent interactions and faster dissolution.
3. Agitation:
Stirring or agitating the solution enhances the dissolution rate by continuously bringing fresh solvent molecules into contact with undissolved solute particles. This prevents the formation of a layer of saturated solution around the solute, which would otherwise hinder further dissolution.
4. Particle Size:
Smaller solute particles dissolve faster than larger ones. The smaller the particles, the greater the surface area exposed to the solvent, leading to a faster dissolution rate.
5. Solvent Properties:
The nature of the solvent plays a crucial role in the dissolution rate. Polar solvents dissolve polar solutes faster, while nonpolar solvents dissolve nonpolar solutes faster. The viscosity of the solvent also influences the dissolution rate; less viscous solvents allow for faster diffusion of solute particles.
Models Describing Dissolution
Several models are employed to describe and predict dissolution rates:
1. Noyes-Whitney Equation:
This equation is a fundamental model used to describe the dissolution rate of a solid in a liquid:
dM/dt = kA(Cs - C)
Where:
dM/dtis the dissolution rate (mass dissolved per unit time)kis the dissolution rate constantAis the surface area of the soluteCsis the saturation solubility of the soluteCis the concentration of the solute in the bulk solution
This equation highlights the importance of surface area and the concentration gradient (Cs - C) in determining the dissolution rate.
2. Hixson-Crowell Cube Root Law:
This model is particularly useful for describing the dissolution of particles that change shape during the dissolution process:
dM/dt = k'A^(1/3)
Where:
k'is a modified dissolution rate constant.
This model accounts for the changes in the particle's size and shape during dissolution.
3. More Complex Models:
For more complex scenarios, more sophisticated models incorporating factors like diffusion through boundary layers, crystal growth, and aggregation are necessary. These models frequently involve numerical simulations and require advanced computational techniques.
Applications of Dissolution Studies
Understanding and controlling the dissolution process is critical in various applications:
-
Pharmaceutical Science: The dissolution rate of a drug significantly affects its bioavailability (the fraction of the drug that reaches the systemic circulation). Dissolution testing is crucial for ensuring consistent drug delivery and efficacy.
-
Environmental Engineering: Dissolution studies are vital for understanding the fate and transport of pollutants in the environment. Knowing the dissolution rate of contaminants helps in designing effective remediation strategies.
-
Materials Science: Dissolution processes are important in material processing, such as the etching of semiconductors and the synthesis of nanoparticles.
-
Food Science: The dissolution of various components in food affects texture, flavor, and nutrient availability.
Conclusion
The dissolution of a solute in a solvent is a complex but fundamental process governed by a combination of thermodynamic and kinetic factors. Understanding these factors and the various models that describe them is crucial across numerous scientific and engineering disciplines. Further research into this field continues to refine our understanding and enables the development of new technologies and applications. The ongoing exploration of dissolution processes promises exciting advancements in various fields, highlighting its enduring importance in scientific inquiry and technological innovation.
Latest Posts
Latest Posts
-
Are Daughter Cells Identical To Each Other In Meiosis
Mar 22, 2025
-
Find An Equation Of The Tangent Plane To The Surface
Mar 22, 2025
-
How To Find The Coordination Number
Mar 22, 2025
-
Functional Unit Of The Kidney Is
Mar 22, 2025
-
Do Fungi Reproduce Asexually Or Sexually
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about An Blan Is Dissolved In A Solvent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
