How To Find The Coordination Number
Muz Play
Mar 22, 2025 · 6 min read
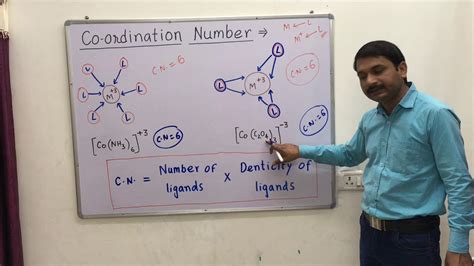
Table of Contents
How to Find the Coordination Number: A Comprehensive Guide
Coordination number, a fundamental concept in chemistry and crystallography, describes the number of atoms, ions, or molecules directly bonded to a central atom in a complex or crystal structure. Understanding how to determine coordination number is crucial for predicting the properties of materials and understanding their behavior. This comprehensive guide will delve into various methods for determining coordination number, encompassing different types of structures and scenarios.
Understanding Coordination Number: The Basics
Before diving into the methods, let's solidify the fundamental understanding of coordination number. It's essential to remember that the coordination number isn't simply about the number of nearby atoms; it's specifically about the number of atoms directly bonded to the central atom. This distinction is key.
Factors influencing Coordination Number:
- Size of the central atom: Larger central atoms can accommodate more ligands (atoms or molecules bonded to the central atom).
- Size of the ligands: Smaller ligands can pack closer around the central atom, allowing for a higher coordination number.
- Charge of the central atom and ligands: Electrostatic interactions play a critical role; stronger interactions can lead to higher coordination numbers.
- Steric factors: The spatial arrangement of ligands can influence how many can fit around the central atom. Bulky ligands will often result in lower coordination numbers due to steric hindrance.
Methods for Determining Coordination Number
Determining the coordination number depends heavily on the type of structure under investigation. We'll explore several common approaches:
1. Coordination Number in Simple Ionic Compounds
In simple ionic compounds like NaCl (sodium chloride), the coordination number is determined by the crystal structure. NaCl adopts a face-centered cubic (fcc) structure.
-
Visual Inspection: By examining the unit cell of NaCl, we can see that each Na⁺ ion is surrounded by six Cl⁻ ions, and each Cl⁻ ion is surrounded by six Na⁺ ions. Therefore, the coordination number for both Na⁺ and Cl⁻ in NaCl is 6.
-
Crystallography Data: X-ray diffraction data provides precise information about the atomic positions within the crystal lattice, allowing for the unambiguous determination of coordination numbers. Analyzing the interatomic distances helps identify the atoms directly bonded to the central atom.
2. Coordination Number in Coordination Complexes
Coordination complexes, involving a central metal ion surrounded by ligands, present a more nuanced scenario. Determining the coordination number here often involves several techniques:
-
Chemical Formula: The chemical formula often provides a clue. For example, in [Co(NH₃)₆]³⁺ (hexaamminecobalt(III) ion), the six ammonia (NH₃) ligands are directly coordinated to the central cobalt(III) ion, giving a coordination number of 6.
-
Spectroscopic Techniques: Techniques like UV-Vis, IR, and NMR spectroscopy provide valuable insights into the bonding and structure of coordination complexes. The spectral data can help identify the number of ligands directly bound to the metal center, revealing the coordination number. For example, the splitting of d-orbitals in transition metal complexes, as observed in UV-Vis spectroscopy, is directly related to the coordination geometry and hence the coordination number.
-
X-ray Crystallography: X-ray crystallography offers the most definitive method for determining the coordination number in coordination complexes. It reveals the precise arrangement of atoms within the complex, providing clear visualization of the bonds between the central metal ion and its ligands.
3. Coordination Number in Metals
Metallic structures involve a complex arrangement of atoms, requiring different approaches to determining coordination number.
-
Crystal Structure: The crystal structure of a metal dictates its coordination number. For example, in body-centered cubic (bcc) structures like that of α-iron, each atom is surrounded by eight nearest neighbors, giving a coordination number of 8. In face-centered cubic (fcc) structures like copper, each atom has twelve nearest neighbors, resulting in a coordination number of 12.
-
Packing Efficiency: The coordination number is directly linked to the packing efficiency of the atoms in the metal lattice. Higher coordination numbers correspond to higher packing efficiency.
-
Computational Methods: Density functional theory (DFT) and other computational methods can be employed to model the metallic structure and accurately predict the coordination number. These computational techniques provide detailed insights into the electronic structure and bonding, strengthening the determination of the coordination number.
4. Coordination Number in Molecular Solids
Molecular solids, formed by intermolecular forces, require a careful consideration of the interactions between molecules to determine the coordination number.
-
Intermolecular Interactions: The strength and nature of the intermolecular interactions (hydrogen bonding, van der Waals forces, etc.) dictate how molecules arrange themselves in the solid state. The number of neighboring molecules significantly interacting with a central molecule determines the coordination number. This assessment is often qualitative.
-
Crystal Structure Analysis: While challenging, X-ray crystallography can help determine the arrangement of molecules in a molecular solid, providing information that facilitates a reasonable estimation of the coordination number.
5. Coordination Number in Complex Structures (e.g., Zeolites)
Complex structures like zeolites possess intricate frameworks. Determining coordination numbers in these situations requires sophisticated techniques:
-
Powder X-ray Diffraction: This technique is particularly useful for characterizing microporous materials like zeolites. By analyzing the diffraction pattern, it is possible to extract structural information and determine the coordination environments of atoms within the framework.
-
Electron Microscopy: High-resolution electron microscopy (HRTEM) provides direct imaging of the atomic arrangement in zeolites, enabling visualization and determination of the coordination number for specific atoms within the framework.
-
Computational Modeling: Modeling the structure of complex materials like zeolites using computational methods is often necessary to refine experimental data and arrive at accurate coordination numbers. These simulations, often DFT-based, provide valuable insights into the structural details of the material.
Advanced Considerations and Applications
Determining coordination number is not merely an academic exercise; it has practical implications in numerous fields:
-
Materials Science: Understanding coordination numbers is essential for designing new materials with specific properties. For example, the coordination number influences the mechanical strength, thermal conductivity, and electronic properties of materials.
-
Catalysis: Coordination numbers play a vital role in heterogeneous catalysis. The coordination environment of active sites on a catalyst surface affects its activity and selectivity.
-
Biochemistry: The coordination numbers of metal ions in enzymes and other biomolecules are crucial to their function. The coordination environment of the metal ion dictates its reactivity and ability to bind to substrates.
-
Geochemistry: Coordination numbers are vital for understanding the structure and stability of minerals. The coordination environment of metal ions in minerals affects their solubility and reactivity in geological processes.
Conclusion
Determining coordination number involves a multifaceted approach dependent on the material's type and complexity. While simple ionic compounds may yield coordination numbers through straightforward visual inspection, more sophisticated techniques like spectroscopy, X-ray crystallography, and computational methods are necessary for complex structures. The importance of understanding coordination number extends to various scientific fields, highlighting its significance in material design, catalysis, biochemistry, and geochemistry. This comprehensive guide has provided a detailed overview of various approaches to determining coordination number, equipping you with the knowledge to navigate this fundamental concept in chemistry and related disciplines. Remember to always consider the context of the material and choose the appropriate methods accordingly. The accurate determination of coordination numbers is crucial for advancing our understanding and manipulation of materials at the atomic and molecular levels.
Latest Posts
Latest Posts
-
What Type Of Energy Is Created By Breaking The Bonds
Mar 22, 2025
-
Glucose Fructose And Galactose Are Examples Of
Mar 22, 2025
-
Give The Symbol For Two Halogens
Mar 22, 2025
-
Why Do Cells Divide Instead Of Growing Larger
Mar 22, 2025
-
C C Bpnd Or C Cl Bond Stronger
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Coordination Number . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
