Give The Symbol For Two Halogens
Muz Play
Mar 22, 2025 · 6 min read
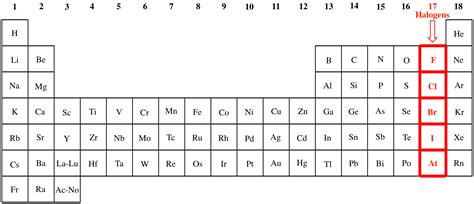
Table of Contents
Give the Symbol for Two Halogens: A Deep Dive into Group 17
The halogens, residing in Group 17 (VIIA) of the periodic table, are a fascinating family of nonmetals known for their reactivity and diverse applications. Understanding their properties, characteristics, and uses is crucial in various scientific fields, from chemistry and materials science to medicine and environmental studies. This article will delve into the world of halogens, focusing on two specific members and providing a comprehensive overview of their symbolic representation, chemical properties, and real-world significance. We'll also explore the broader context of halogen chemistry, touching upon their trends and applications.
The Symbols: Unveiling Chlorine (Cl) and Iodine (I)
Two prominent members of the halogen family are chlorine (Cl) and iodine (I). Their symbols, derived from their Latin names (chlorum and iodum respectively), are fundamental to chemical notation and communication. Understanding these symbols is the first step in comprehending their role in chemical reactions and compounds.
Chlorine (Cl): The Versatile Disinfectant
Chlorine, represented by the symbol Cl, is a yellowish-green gas at room temperature. Its pungent and irritating odor is easily recognizable. While its gaseous form is hazardous, chlorine's versatility and reactivity make it indispensable in various applications.
Key Properties and Uses of Chlorine:
-
Disinfection: Chlorine's potent disinfecting properties are perhaps its most well-known attribute. It's extensively used in water treatment plants to kill harmful bacteria, viruses, and other microorganisms, ensuring safe drinking water for millions. This application significantly impacts public health and sanitation.
-
Bleach Production: Chlorine is a critical component in the production of household bleach (sodium hypochlorite). Bleach's bleaching and disinfecting abilities rely on chlorine's oxidizing power, making it a staple in cleaning and hygiene practices.
-
Chemical Synthesis: Chlorine plays a crucial role in the chemical industry as a reactant in numerous syntheses. It's used in the production of plastics (PVC), solvents, and various other chemicals. The versatility of chlorine in chemical synthesis contributes to the manufacturing of countless products.
-
Pharmaceuticals: While chlorine itself isn't directly used in many pharmaceuticals, its compounds are essential components in the synthesis of numerous medications. Its role in creating various pharmaceutical intermediates is quite significant.
-
Pesticides: Certain chlorine-containing compounds are used in pesticides, though this application is becoming less common due to environmental concerns. The debate around the usage of chlorine-based pesticides highlights the need for responsible application and sustainable alternatives.
Iodine (I): Essential for Thyroid Function
Iodine, represented by the symbol I, is a dark gray, lustrous solid at room temperature that sublimes (transitions directly from solid to gas) upon heating. Unlike chlorine's widespread industrial uses, iodine's importance lies primarily in its biological role.
Key Properties and Uses of Iodine:
-
Thyroid Hormone Production: Iodine is an essential micronutrient, critical for the proper functioning of the thyroid gland. The thyroid gland uses iodine to produce thyroid hormones (thyroxine (T4) and triiodothyronine (T3)) that regulate metabolism, growth, and development. Iodine deficiency can lead to serious health problems such as goiter, hypothyroidism, and developmental issues.
-
Medical Applications: Iodine's antiseptic properties have led to its use as an antiseptic and disinfectant in various medical applications. Iodine solutions are used to sterilize wounds and skin before surgical procedures. Iodine-based contrast agents are also used in medical imaging.
-
Nutrition: Iodine is added to table salt (iodized salt) to prevent iodine deficiency. This crucial public health initiative significantly reduces the incidence of iodine-related disorders in populations with iodine-deficient diets.
-
Industrial Applications: While less prevalent than chlorine's, iodine does have some industrial applications. It's used in the production of certain dyes, pharmaceuticals, and catalysts.
Exploring the Broader Halogen Family
Beyond chlorine and iodine, the halogen family includes fluorine (F), bromine (Br), and astatine (At). Each halogen shares certain characteristic properties, though their specific properties vary depending on their position in the periodic table.
General Properties of Halogens:
-
High Electronegativity: Halogens have high electronegativity, meaning they strongly attract electrons in chemical bonds. This high electronegativity drives their reactivity.
-
Reactivity: Halogens are highly reactive, readily forming ionic compounds with metals and covalent compounds with other nonmetals. Their reactivity generally decreases down the group (fluorine being the most reactive, astatine the least).
-
Seven Valence Electrons: Halogens all have seven valence electrons, meaning they need only one more electron to achieve a stable octet configuration. This drives their tendency to gain an electron in chemical reactions.
-
Diatomic Molecules: Halogens exist as diatomic molecules in their elemental form (e.g., Cl₂, I₂, F₂, Br₂). This means they exist as pairs of atoms bonded together.
-
Oxidizing Agents: Because of their high electronegativity, halogens act as strong oxidizing agents, readily accepting electrons from other substances. This oxidizing ability underlies their applications in disinfection and bleaching.
Trends in Halogen Properties:
As we move down the halogen group from fluorine to astatine, several trends become apparent:
-
Decreasing Electronegativity: Electronegativity decreases as we move down the group. Fluorine is the most electronegative element.
-
Decreasing Reactivity: Reactivity also decreases down the group. Fluorine is the most reactive halogen, while astatine is the least.
-
Increasing Atomic Radius: Atomic radius increases as we move down the group, reflecting the addition of electron shells.
-
Increasing Melting and Boiling Points: Melting and boiling points generally increase down the group due to increasing intermolecular forces.
Environmental Considerations and the Halogens
The use of halogens, particularly chlorine and its compounds, has raised environmental concerns. Chlorofluorocarbons (CFCs), once widely used as refrigerants and propellants, were found to deplete the ozone layer. The Montreal Protocol, an international treaty, phased out the production and consumption of ozone-depleting substances, highlighting the importance of responsible halogen usage.
Moreover, certain organohalogen compounds, such as dioxins and PCBs (polychlorinated biphenyls), are persistent organic pollutants (POPs) that persist in the environment and pose health risks. Efforts are ongoing to mitigate the release and impact of these harmful compounds.
Conclusion: The Importance of Understanding Halogen Symbols and Properties
The symbols for chlorine (Cl) and iodine (I) are fundamental to understanding the chemical behavior and applications of these important elements. Their individual properties, along with the general trends within the halogen group, contribute significantly to their diverse uses in various industries and their crucial roles in biological systems. It's vital to consider the environmental impacts of halogen usage and to promote responsible applications while exploring sustainable alternatives. Further research and development are crucial to harness the benefits of halogens while mitigating their potential risks. The continuous study of halogens ensures their safe and effective utilization across diverse fields, contributing to scientific advancement and societal well-being. This detailed exploration provides a strong foundation for understanding these vital elements and their significance in the world around us.
Latest Posts
Latest Posts
-
The Quantity Of Matter In An Object
Mar 22, 2025
-
Converting From Rectangular To Spherical Coordinates
Mar 22, 2025
-
An Area Defined By Some Common Characteristics
Mar 22, 2025
-
Is Mg A Metal Nonmetal Or Metalloid
Mar 22, 2025
-
Describe Internal Factors Of Decision Making
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Give The Symbol For Two Halogens . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
