Is Mg A Metal Nonmetal Or Metalloid
Muz Play
Mar 22, 2025 · 6 min read
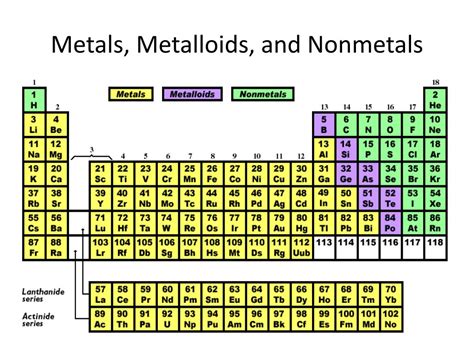
Table of Contents
Is Mg a Metal, Nonmetal, or Metalloid? A Deep Dive into Magnesium's Properties
Magnesium (Mg), element number 12 on the periodic table, is a fascinating element with a wide array of applications. But where does it fit in the broad classification of elements? Is it a metal, a nonmetal, or a metalloid? This comprehensive guide will delve into magnesium's properties to definitively answer this question and explore its unique characteristics.
Understanding the Classification of Elements
Before we classify magnesium, let's briefly revisit the three major categories of elements:
1. Metals
Metals are typically good conductors of electricity and heat, possess a lustrous appearance, are malleable (can be hammered into sheets), and are ductile (can be drawn into wires). They tend to lose electrons easily, forming positive ions (cations). Examples include iron (Fe), copper (Cu), and gold (Au).
2. Nonmetals
Nonmetals are generally poor conductors of electricity and heat, lack a metallic luster, and are typically brittle. They tend to gain electrons easily, forming negative ions (anions). Examples include oxygen (O), chlorine (Cl), and sulfur (S).
3. Metalloids (Semimetals)
Metalloids exhibit properties intermediate between metals and nonmetals. Their conductivity can vary depending on factors like temperature and pressure. They often have a semi-metallic luster. Examples include silicon (Si), germanium (Ge), and arsenic (As).
Magnesium: A Definitive Metal
Without a doubt, magnesium is a metal. It exhibits all the key characteristics of metals:
1. Excellent Conductivity:
Magnesium is a relatively good conductor of both electricity and heat. This property is crucial for its use in various applications, including alloys and electronics. Its conductivity stems from the ease with which its valence electrons can move freely within its metallic structure.
2. Metallic Luster and Appearance:
Magnesium possesses a silvery-white, lustrous appearance. This shiny surface is characteristic of most metals and is a direct result of the interaction of light with its electron sea. The surface can tarnish over time due to oxidation, but the underlying metallic luster remains.
3. Malleability and Ductility:
While not as malleable or ductile as some other metals like gold, magnesium is still capable of being shaped and formed. It can be rolled into sheets and drawn into wires, albeit with more difficulty than highly malleable metals. This property is vital for its use in manufacturing processes.
4. Electron Loss and Ion Formation:
Magnesium readily loses its two valence electrons to achieve a stable electron configuration, forming a Mg²⁺ cation. This tendency to lose electrons is a hallmark characteristic of metals and explains its reactivity with various substances. This reactivity is exploited in many of its applications, from sacrificial anodes to chemical reactions.
Detailed Properties of Magnesium Highlighting its Metallic Nature
Let's delve deeper into specific properties of magnesium which further solidify its classification as a metal:
1. Density:
Magnesium has a relatively low density compared to many other metals. This makes it a lightweight metal, which is a significant advantage in various applications such as aerospace and automotive industries, where weight reduction is crucial for fuel efficiency and performance.
2. Strength and Hardness:
While not as strong or hard as steel, magnesium alloys exhibit reasonable strength and hardness. The addition of alloying elements further enhances these properties, making them suitable for structural components in various applications.
3. Melting and Boiling Points:
Magnesium possesses a relatively low melting point compared to many other metals, making it relatively easy to melt and cast. This low melting point, along with its other properties, makes it suitable for various casting processes. Its boiling point is significantly higher, indicating strong metallic bonding.
4. Reactivity:
Magnesium is a moderately reactive metal. It readily reacts with acids, releasing hydrogen gas. This reactivity is exploited in certain chemical reactions and is also a reason for its use as a sacrificial anode in corrosion protection. Its reactivity, however, is still distinctly metallic, as opposed to the largely unreactive nature of many nonmetals.
5. Crystal Structure:
Magnesium has a hexagonal close-packed (HCP) crystal structure. This specific arrangement of atoms is common in many metals and contributes to the material's overall physical properties. This structured arrangement, again, is typical of metals and further supports its metallic nature.
Applications of Magnesium: A Testament to its Metallic Properties
The diverse applications of magnesium are a strong testament to its metallic properties. These applications wouldn't be possible if magnesium were a nonmetal or metalloid:
- Aerospace: Magnesium alloys are used extensively in aircraft and spacecraft construction due to their lightweight yet reasonably strong nature.
- Automotive: Similar to aerospace, the automotive industry utilizes magnesium alloys for components requiring lightweight yet durable materials.
- Electronics: Magnesium's electrical conductivity makes it suitable for electronic components and applications.
- Biomedical: Magnesium is essential for human health and is also used in biodegradable implants and devices.
- Chemical Industry: Magnesium's reactivity is harnessed in various chemical processes and reactions.
- Pyrotechnics: Magnesium's ability to burn brightly and produce intense light makes it a key component in fireworks and flares.
Debunking Misconceptions: Why Magnesium is NOT a Metalloid or Nonmetal
Some individuals may mistakenly believe magnesium to have characteristics of metalloids or nonmetals. However, a thorough understanding of its properties definitively rules out these classifications. Magnesium's consistent display of metallic traits makes such misclassifications inaccurate:
-
Conductivity: Magnesium's excellent conductivity is a clear indicator of its metallic nature. Metalloids possess variable conductivity, often dependent on external factors. Nonmetals are generally poor conductors.
-
Appearance: Magnesium's metallic luster strongly differentiates it from nonmetals, which typically lack a shiny appearance. While some metalloids may exhibit a semi-metallic luster, magnesium's is far more pronounced and characteristic of metals.
-
Reactivity: Magnesium's reactivity aligns with the typical behavior of metals, forming positive ions. Nonmetals tend to gain electrons, forming negative ions. Metalloids show a range of reactivity.
-
Crystal structure and bonding: Magnesium's HCP crystal structure and metallic bonding are definitive hallmarks of its metallic nature.
Conclusion: Magnesium - A Metal Through and Through
In conclusion, there is no ambiguity. Magnesium is unequivocally a metal. Its excellent electrical and thermal conductivity, metallic luster, malleability (to an extent), ductility, reactivity in forming positive ions, and its broad range of applications directly linked to these metallic properties, conclusively place it within the metal category on the periodic table. Any suggestion to the contrary is incorrect and unsupported by scientific evidence. Understanding magnesium’s metallic nature is crucial for appreciating its versatility and wide-ranging applications in diverse fields.
Latest Posts
Latest Posts
-
Pie Chart Of The Cell Cycle
Mar 23, 2025
-
A Que Temperatura Se Congela El Agua En Grados Fahrenheit
Mar 23, 2025
-
Blood Flow Through The Capillary Beds Is Regulated By
Mar 23, 2025
-
5 Stages Of The Listening Process
Mar 23, 2025
-
The Crural Region Of The Body Is The
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Is Mg A Metal Nonmetal Or Metalloid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
