A Que Temperatura Se Congela El Agua En Grados Fahrenheit
Muz Play
Mar 23, 2025 · 5 min read
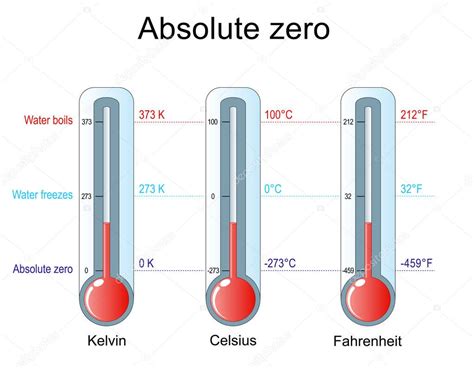
Table of Contents
At What Temperature Does Water Freeze in Fahrenheit? A Deep Dive into Freezing Points
Water, the elixir of life, exhibits fascinating properties, and one of the most fundamental is its freezing point. While many know that water freezes at 32 degrees Fahrenheit, understanding the intricacies behind this seemingly simple phenomenon requires a deeper exploration. This comprehensive article delves into the science of water freezing, exploring the factors that can influence this temperature, the implications for various applications, and debunking common misconceptions.
Understanding the Freezing Point of Water
The freezing point of water, the temperature at which liquid water transitions into solid ice, is 32 degrees Fahrenheit (0 degrees Celsius or 273.15 Kelvin). This temperature represents the equilibrium point where the rate of water molecules transitioning from liquid to solid equals the rate of molecules transitioning from solid to liquid. At temperatures below 32°F, the kinetic energy of water molecules decreases to a point where the attractive forces between them become dominant, leading to the formation of a crystalline structure – ice.
Factors Influencing the Freezing Point
While 32°F is the standard freezing point of pure water at sea level, several factors can subtly alter this temperature:
-
Pressure: Increased pressure lowers the freezing point of water. This is an unusual property, as most substances have their freezing points raised by increased pressure. This phenomenon is crucial in understanding processes like ice skating, where the pressure exerted by the skate blades on the ice slightly lowers the freezing point, causing a thin layer of water to form, facilitating smoother gliding.
-
Impurities: The presence of dissolved substances, like salts or sugars, in water lowers its freezing point. This is why salt is used to de-ice roads and sidewalks in winter. The salt dissolves in the water, lowering the freezing point below the ambient temperature, causing the ice to melt. The extent of freezing point depression is directly proportional to the concentration of dissolved impurities – a higher concentration leads to a greater decrease in the freezing point.
-
Altitude: At higher altitudes, the atmospheric pressure is lower, which slightly raises the boiling point of water but also slightly lowers its freezing point. This effect is minimal at moderate altitudes but becomes more noticeable at significantly higher elevations.
-
Supercooling: Under specific conditions, water can remain liquid even below its freezing point. This phenomenon, known as supercooling, occurs when there are few nucleation sites for ice crystal formation. Nucleation sites are imperfections or irregularities in the water, such as dust particles or scratches on the container, that provide a surface for ice crystals to begin forming. In the absence of these sites, water can remain in a metastable supercooled state until disturbed, at which point it will rapidly freeze.
Applications and Implications of Water's Freezing Point
The freezing point of water has profound implications across various fields and applications:
Food Preservation:
Freezing food is a common method of preservation that relies on the principle of lowering the water activity within the food, thus inhibiting the growth of microorganisms and slowing down enzymatic reactions. Knowing the freezing point of water is crucial for effective food freezing and storage. Faster freezing generally preserves food quality better, as larger ice crystals can damage cell structures.
Cryogenics:
Cryogenics utilizes extremely low temperatures to study materials and biological samples. Understanding the freezing point of water, and the behavior of water at sub-zero temperatures, is fundamental to designing and operating cryogenic equipment and processes.
Meteorology and Climatology:
The freezing point of water is paramount in understanding weather patterns and climate change. The formation of ice crystals in clouds plays a crucial role in precipitation, and changes in freezing temperatures can significantly impact ecosystems and agriculture.
Civil Engineering:
The freezing and thawing of water within concrete and other construction materials can lead to significant damage over time. Understanding the freezing point of water and its impact on material properties is essential for designing durable and reliable structures in regions with freezing temperatures.
Debunking Common Misconceptions
Several misconceptions surround the freezing point of water:
-
Myth: Adding salt to water always makes it freeze faster. Fact: Adding salt lowers the freezing point, meaning the water will freeze at a lower temperature. While it might seem like it freezes faster because the initial freezing temperature is lower, the overall freezing process actually takes longer due to the reduced freezing point.
-
Myth: Ice melts faster in warm water. Fact: The rate of melting depends more on the temperature difference between the ice and the surrounding environment. While warm water has a greater capacity to absorb heat and melt ice, the temperature difference determines the speed. A larger temperature difference leads to faster melting, regardless of whether the water is warm or only slightly above freezing.
-
Myth: All water freezes at exactly 32°F. Fact: As discussed earlier, various factors can influence the freezing point of water, including pressure, impurities, and altitude. Pure water at standard atmospheric pressure will freeze at 32°F, but deviations are possible under different conditions.
Conclusion
The seemingly simple freezing point of water at 32°F is a complex phenomenon with far-reaching consequences across various scientific disciplines and everyday applications. Understanding the factors that influence this point and the implications for different processes is crucial for scientific advancement and technological innovation. From preserving food to understanding climate change, a thorough understanding of water's freezing point is essential for navigating our world. Further research into the subtleties of ice formation and the behavior of water under diverse conditions continues to unveil new insights into this fundamental property of the most abundant substance on Earth. The freezing point of water isn't merely a number; it's a gateway to understanding the intricate processes of our world.
Latest Posts
Latest Posts
-
Transfer Function Of An Rc Circuit
Mar 25, 2025
-
How Do You Find The Molar Mass Of A Gas
Mar 25, 2025
-
Examples Include Oils Waxes And Butters
Mar 25, 2025
-
Common Particles With Charge Of 2
Mar 25, 2025
-
What Does The Top Command Do In Linux
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about A Que Temperatura Se Congela El Agua En Grados Fahrenheit . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
