How Do You Find The Molar Mass Of A Gas
Muz Play
Mar 25, 2025 · 7 min read
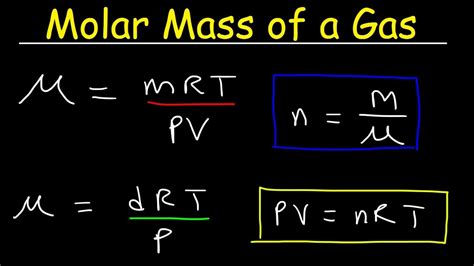
Table of Contents
How Do You Find the Molar Mass of a Gas? A Comprehensive Guide
Determining the molar mass of a gas is a fundamental concept in chemistry with applications ranging from environmental monitoring to industrial process control. Understanding how to calculate this crucial value opens doors to a deeper comprehension of gas behavior and its role in various chemical reactions. This comprehensive guide will equip you with the knowledge and techniques to accurately determine the molar mass of a gas, exploring various methods and their underlying principles.
Understanding Molar Mass
Before delving into the methods, let's establish a clear understanding of what molar mass actually represents. Molar mass is defined as the mass of one mole of a substance. A mole is a unit representing Avogadro's number (approximately 6.022 x 10<sup>23</sup>) of particles, whether they are atoms, molecules, or ions. The molar mass is typically expressed in grams per mole (g/mol). For example, the molar mass of oxygen gas (O<sub>2</sub>) is approximately 32 g/mol because one mole of O<sub>2</sub> contains 6.022 x 10<sup>23</sup> molecules of O<sub>2</sub>, and the mass of that many molecules is 32 grams.
Methods for Determining Molar Mass of a Gas
Several methods can be employed to determine the molar mass of a gas, each relying on different properties and relationships governed by the ideal gas law or variations thereof. The most common methods include:
1. Using the Ideal Gas Law (PV=nRT)
The ideal gas law, PV = nRT, is the cornerstone of many gas law calculations, including molar mass determination. Let's break down each variable:
- P: Pressure of the gas (usually in atmospheres, atm)
- V: Volume of the gas (usually in liters, L)
- n: Number of moles of the gas (mol)
- R: Ideal gas constant (0.0821 L·atm/mol·K)
- T: Temperature of the gas (in Kelvin, K)
To find the molar mass (M), we need to rearrange the ideal gas law. We know that the number of moles (n) is equal to the mass (m) of the gas divided by its molar mass (M): n = m/M. Substituting this into the ideal gas law, we get:
PV = (m/M)RT
Now, we can rearrange this equation to solve for molar mass (M):
M = (mRT) / (PV)
This equation allows us to calculate the molar mass (M) if we know the mass (m), pressure (P), volume (V), and temperature (T) of the gas. Remember to use consistent units throughout the calculation to ensure accurate results. The accuracy of this method hinges on how closely the gas behaves ideally. Deviations from ideal behavior, particularly at high pressures and low temperatures, can introduce significant errors.
Example: A 1.20 g sample of an unknown gas occupies 0.500 L at 25°C and 1.00 atm. What is its molar mass?
- Convert Celsius to Kelvin: 25°C + 273.15 = 298.15 K
- Substitute values into the equation: M = (1.20 g × 0.0821 L·atm/mol·K × 298.15 K) / (1.00 atm × 0.500 L)
- Calculate: M ≈ 58.9 g/mol
2. Using Density and the Ideal Gas Law
The density (ρ) of a gas is defined as its mass (m) per unit volume (V): ρ = m/V. We can substitute this into the rearranged ideal gas law from the previous section:
M = (ρRT) / P
This equation provides a convenient method for determining molar mass if the density of the gas is known along with its pressure and temperature. This method is particularly useful when the mass of the gas sample is not directly measured.
Example: The density of a gas at 27°C and 1 atm pressure is 1.96 g/L. What is its molar mass?
- Convert Celsius to Kelvin: 27°C + 273.15 = 300.15 K
- Substitute values into the equation: M = (1.96 g/L × 0.0821 L·atm/mol·K × 300.15 K) / 1 atm
- Calculate: M ≈ 48.0 g/mol
3. Dumas Method
The Dumas method is a classic experimental technique for determining the molar mass of a volatile liquid. It involves vaporizing a known mass of the liquid and measuring the volume of the vapor it occupies under known conditions of temperature and pressure. The molar mass is then calculated using the ideal gas law. This is an indirect method for determining the molar mass of a gas. Essentially, you're determining the molar mass of the liquid, but once it's vaporized, it behaves as a gas.
The procedure generally involves:
- Weighing an empty flask: This provides a baseline mass.
- Adding a small amount of the liquid: The exact mass of the liquid added needs to be recorded.
- Heating the flask: This vaporizes the liquid, filling the flask with the gaseous form.
- Cooling and weighing: Once cooled, the flask and its condensed vapor are weighed again. The difference represents the mass of the vaporized liquid.
- Measuring the volume and temperature: The volume of the flask is either known or measured, and the temperature of the vapor is recorded.
- Applying the ideal gas law: Use the mass of the vapor, volume, temperature, and pressure (atmospheric pressure) to calculate the molar mass using the equation derived from the ideal gas law.
Limitations of the Dumas Method: The method relies on the complete vaporization of the liquid and the assumption that the vapor behaves ideally. Errors can arise from incomplete vaporization or from deviations from ideal gas behavior.
4. Effusion and Diffusion Methods (Graham's Law)
Graham's Law of Effusion and Diffusion relates the rates of effusion or diffusion of two gases to their molar masses. Effusion is the escape of gas molecules through a small hole, while diffusion is the spreading of gas molecules throughout a space. Graham's Law states that the rate of effusion or diffusion of a gas is inversely proportional to the square root of its molar mass:
Rate<sub>1</sub> / Rate<sub>2</sub> = √(M<sub>2</sub> / M<sub>1</sub>)
If you know the rate of effusion or diffusion of a known gas and an unknown gas, and the molar mass of the known gas, you can use Graham's Law to determine the molar mass of the unknown gas. This method is less commonly used in comparison to the ideal gas law-based methods.
Factors Affecting Accuracy
Several factors can affect the accuracy of molar mass determination:
- Ideal Gas Behavior: The ideal gas law assumes that gas molecules have negligible volume and do not interact with each other. Real gases deviate from ideal behavior, particularly at high pressures and low temperatures. Using the Van der Waals equation or other equations of state can improve accuracy for non-ideal gases.
- Measurement Errors: Inaccurate measurements of pressure, volume, temperature, and mass can lead to significant errors in the calculated molar mass. Precise measurement techniques are essential.
- Purity of the Gas Sample: Impurities in the gas sample can affect the calculated molar mass. It is crucial to use a pure gas sample to ensure accurate results.
- Leaks in the Apparatus: In experimental setups, leaks can cause loss of gas, leading to inaccurate measurements of volume and mass. Careful sealing of the apparatus is necessary.
Conclusion
Determining the molar mass of a gas is a vital skill in chemistry, with applications in diverse fields. Several methods, primarily relying on the ideal gas law and its variations, allow for the calculation of molar mass. Accurate results depend on precise measurements, consideration of non-ideal behavior (where applicable), and a pure gas sample. Understanding the underlying principles and potential sources of error is crucial for obtaining reliable and meaningful results. By employing the methods and considerations described in this guide, you can confidently determine the molar mass of a gas and deepen your understanding of gas behavior and its role in chemical systems.
Latest Posts
Latest Posts
-
Organelles In Eukaryotic Cells Answer Key
Mar 28, 2025
-
Lewis Dot Diagram For Ionic Bonding Between Li And F
Mar 28, 2025
-
What Is The Relationship Between Avogadros Number And The Mole
Mar 28, 2025
-
What Are The Building Blocks For Fats
Mar 28, 2025
-
The Si Unit Of Energy Is The
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Do You Find The Molar Mass Of A Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
