What Is The Relationship Between Avogadro's Number And The Mole
Muz Play
Mar 28, 2025 · 5 min read
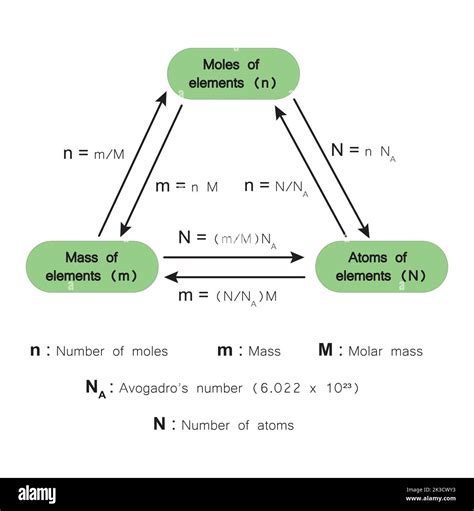
Table of Contents
What is the Relationship Between Avogadro's Number and the Mole?
Understanding the relationship between Avogadro's number and the mole is fundamental to mastering stoichiometry and various other chemical concepts. This relationship forms the cornerstone of quantitative chemistry, allowing us to bridge the gap between the microscopic world of atoms and molecules and the macroscopic world of laboratory measurements. This article will delve deep into this crucial connection, exploring its significance and implications in chemical calculations and analysis.
Defining the Mole: The Chemist's Counting Unit
Before exploring the link with Avogadro's number, let's clarify what a mole actually is. A mole (mol) is simply a unit of measurement, much like a dozen (12) or a gross (144). However, instead of representing a dozen eggs or a gross of pencils, a mole represents a specific number of particles – atoms, molecules, ions, or any other elementary entities. This specific number is precisely what Avogadro's number defines.
The mole is incredibly useful because it allows chemists to work with manageable quantities of substances that contain vast numbers of atoms or molecules. Imagine trying to weigh out individual atoms! The mole provides a practical way to relate the microscopic world to macroscopic measurements, such as mass and volume.
The Importance of the Mole in Chemistry
The mole plays a pivotal role in several key areas of chemistry, including:
-
Stoichiometry: The mole is essential for performing stoichiometric calculations, which involve determining the relative amounts of reactants and products in a chemical reaction. Balanced chemical equations use moles as the unit of measure to quantify the reactants and products.
-
Concentration calculations: Molarity, a common unit of concentration, is defined as the number of moles of solute per liter of solution. Understanding moles is crucial for preparing solutions of specific concentrations.
-
Gas laws: The ideal gas law, PV = nRT, uses the number of moles (n) to relate pressure (P), volume (V), temperature (T), and the ideal gas constant (R).
-
Thermochemistry: Many thermodynamic calculations, such as enthalpy changes, involve moles to express the amount of substance involved in a reaction.
Avogadro's Number: The Bridge Between the Micro and Macro Worlds
Avogadro's number (N<sub>A</sub>) is defined as the number of constituent particles (atoms, molecules, ions, etc.) contained in one mole of a substance. This number is approximately 6.022 x 10<sup>23</sup>. It's an incredibly large number, reflecting the vastness of the number of particles in even small amounts of matter.
It's important to note that Avogadro's number is not a precisely calculated value but rather a measured constant. It’s derived from experimental measurements relating the macroscopic properties of substances to their microscopic composition. Different methods exist to determine Avogadro's number, but they all converge on a remarkably consistent value.
The Origin of Avogadro's Number
The constant is named after Amedeo Avogadro, an Italian scientist whose hypothesis (Avogadro's hypothesis) stated that equal volumes of gases at the same temperature and pressure contain the same number of particles, regardless of the type of gas. While Avogadro himself didn't calculate the number, his hypothesis laid the groundwork for its eventual determination.
Determining this massive number accurately involved precise measurements of physical constants, including the Faraday constant and the charge of an electron. The modern value of Avogadro's number is a result of refined experimental techniques and ongoing scientific advancements.
The Inseparable Relationship: Avogadro's Number and the Mole
The relationship between Avogadro's number and the mole is fundamentally definitional. They are inextricably linked:
-
One mole of any substance contains Avogadro's number (6.022 x 10<sup>23</sup>) of constituent particles. This applies to atoms, molecules, ions, formula units – any type of chemical entity.
-
Avogadro's number provides the conversion factor between the number of moles and the number of particles. To convert from moles to the number of particles, multiply the number of moles by Avogadro's number. Conversely, to convert from the number of particles to moles, divide the number of particles by Avogadro's number.
This relationship is expressed mathematically as:
Number of particles = Number of moles x Avogadro's number
or
Number of moles = Number of particles / Avogadro's number
Practical Applications and Examples
Let's illustrate the practical applications of this relationship with a few examples:
Example 1: How many atoms are there in 2 moles of carbon?
- Using the formula: Number of atoms = Number of moles x Avogadro's number
- Number of atoms = 2 mol x 6.022 x 10<sup>23</sup> atoms/mol
- Number of atoms = 1.204 x 10<sup>24</sup> atoms
Example 2: How many moles are present in 3.011 x 10<sup>23</sup> molecules of water?
- Using the formula: Number of moles = Number of particles / Avogadro's number
- Number of moles = 3.011 x 10<sup>23</sup> molecules / 6.022 x 10<sup>23</sup> molecules/mol
- Number of moles = 0.5 mol
Beyond Simple Calculations: Deeper Implications
The relationship between Avogadro's number and the mole extends far beyond simple conversions. It underpins our understanding of:
-
Molar mass: The molar mass of a substance is the mass of one mole of that substance in grams. It's numerically equal to the atomic or molecular weight of the substance. This connection allows us to convert between mass and moles, a crucial step in many chemical calculations.
-
Empirical and molecular formulas: Avogadro's number allows us to determine the empirical and molecular formulas of compounds using experimental data on the mass composition of the substance.
-
Gas stoichiometry: The ideal gas law, combined with the mole concept and Avogadro's number, allows for quantitative calculations involving gaseous reactants and products.
Conclusion: A Cornerstone of Chemistry
The relationship between Avogadro's number and the mole is not just a mathematical formula; it's a fundamental concept that unites the microscopic and macroscopic worlds in chemistry. Understanding this relationship is crucial for performing quantitative analyses, interpreting chemical reactions, and building a strong foundation in chemistry. From stoichiometric calculations to gas laws and concentration determinations, the mole and Avogadro's number are essential tools for any chemist, ensuring the accurate and reliable quantification of matter at both the atomic and bulk levels. Mastering this concept unlocks a deeper understanding of chemical reactions, properties, and processes, providing a solid base for further exploration in this fascinating field.
Latest Posts
Latest Posts
-
What Holds An Ionic Bond Together
Mar 31, 2025
-
Genotype And Phenotype Punnett Square Examples
Mar 31, 2025
-
Equation Relating Electric Field And Voltage
Mar 31, 2025
-
Does A Plant Cell Have Dna
Mar 31, 2025
-
Concept Map Sympathetic And Parasympathetic Responses
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Relationship Between Avogadro's Number And The Mole . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
