Lewis Dot Diagram For Ionic Bonding Between Li And F
Muz Play
Mar 28, 2025 · 5 min read
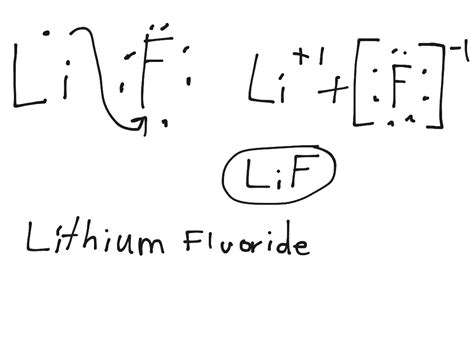
Table of Contents
Lewis Dot Diagrams: Unveiling the Ionic Bond Between Lithium and Fluorine
The world of chemistry is built upon fundamental interactions between atoms, leading to the formation of molecules and compounds. One of the most crucial of these interactions is ionic bonding, a powerful force driven by the electrostatic attraction between oppositely charged ions. This article delves deep into the ionic bonding between lithium (Li) and fluorine (F), utilizing Lewis dot diagrams to visualize and understand this fundamental chemical process. We'll explore the electron transfer, the resulting charges, and the overall stability achieved through this ionic interaction.
Understanding Lewis Dot Diagrams
Before we embark on our journey into the Li-F ionic bond, let's solidify our understanding of Lewis dot diagrams. These diagrams, also known as electron dot structures, are simple yet powerful tools used to represent the valence electrons of an atom. Valence electrons are the electrons residing in the outermost shell of an atom and are primarily involved in chemical bonding.
Key Features of Lewis Dot Diagrams:
- The Atomic Symbol: The symbol of the element is placed in the center.
- Valence Electrons: Dots surrounding the symbol represent the valence electrons. Each dot represents one electron.
- Electron Placement: Electrons are placed individually around the symbol until all valence electrons are represented. Pairing of electrons occurs only after each side has a single electron.
For example, let's consider lithium (Li), an alkali metal with an atomic number of 3. Its electron configuration is 1s²2s¹. This means it has one electron in its valence shell (the 2s subshell). Therefore, the Lewis dot diagram for Li would be:
Li •
Fluorine (F), a halogen with an atomic number of 9, has an electron configuration of 1s²2s²2p⁵. It possesses seven valence electrons (two in the 2s and five in the 2p subshells). Its Lewis dot diagram is:
•• F ••
The Ionic Bond Formation Between Lithium and Fluorine
Lithium, with its single valence electron, readily loses this electron to achieve a stable electron configuration resembling that of the noble gas helium (He), which has a completely filled outer shell. This loss of an electron results in a positively charged lithium ion (Li⁺).
Conversely, fluorine, with its seven valence electrons, readily gains one electron to achieve a stable electron configuration resembling that of the noble gas neon (Ne), with a completely filled outer shell. This gain of an electron results in a negatively charged fluoride ion (F⁻).
The electrostatic attraction between the positively charged lithium ion (Li⁺) and the negatively charged fluoride ion (F⁻) constitutes the ionic bond, forming the ionic compound lithium fluoride (LiF).
Step-by-Step Visualizing with Lewis Dot Diagrams
Let's illustrate the ionic bond formation between Li and F using Lewis dot diagrams:
-
Start with the individual atoms: Begin with the Lewis dot diagrams for Li and F, as shown previously.
-
Lithium loses an electron: Lithium's single valence electron is transferred to fluorine. This is represented by removing the dot from Li and showing Li as a positive ion (Li⁺), now without any valence electrons.
-
Fluorine gains an electron: The electron transferred from lithium is added to the fluorine atom's Lewis dot diagram, completing its octet (eight valence electrons). Fluorine now becomes a negative ion (F⁻).
-
Formation of the ionic compound: The electrostatic attraction between Li⁺ and F⁻ forms the ionic compound LiF. The Lewis dot structure doesn't directly show the ionic bond, but it represents the charged ions that are electrostatically attracted to each other. The ionic bond is represented by the brackets, typically showing the charges: [Li⁺][F⁻].
Here's a visual representation of this process:
Li • + •• → [Li⁺] + [F⁻] F ••
Octet Rule and Stability
The driving force behind ionic bonding is the achievement of a stable electron configuration, typically involving a complete outer shell of eight electrons (octet rule). Both lithium and fluorine achieve this stability by participating in the ionic bond.
Lithium, by losing its single valence electron, achieves the stable electron configuration of helium (1s²). Fluorine, by gaining one electron, achieves the stable electron configuration of neon (1s²2s²2p⁶). This attainment of stability is the key reason why the ionic bond between Li and F is energetically favorable and strong.
Properties of Ionic Compounds
Ionic compounds, such as LiF, exhibit several characteristic properties:
-
High Melting and Boiling Points: The strong electrostatic forces between the oppositely charged ions require significant energy to overcome, resulting in high melting and boiling points.
-
Crystalline Structure: Ionic compounds form crystalline structures with a regular, repeating arrangement of ions to maximize electrostatic attractions and minimize repulsions.
-
Brittle Nature: The rigid arrangement of ions in the crystal lattice makes ionic compounds brittle; a slight shift in the crystal lattice can cause like charges to align, leading to repulsion and fracture.
-
Conductivity in Molten State or Solution: While solid ionic compounds are poor conductors of electricity, they become good conductors when molten or dissolved in water, as the ions become mobile and can carry electric charge.
Beyond the Basics: Exploring Further
The simple Lewis dot diagram provides a fundamental understanding of ionic bonding in LiF. However, a deeper dive reveals nuances that go beyond this simplified representation.
-
Electrostatic Potential: The attraction between Li⁺ and F⁻ isn't uniform; the electrostatic potential varies within the ionic bond. Advanced calculations and simulations are employed to accurately depict this potential.
-
Lattice Energy: The lattice energy is the energy released when gaseous ions combine to form one mole of a solid ionic compound. It quantifies the strength of the ionic bond. The high lattice energy in LiF demonstrates the strength of the ionic interaction.
-
Polarity: The Li-F bond is highly polar, with a significant difference in electronegativity between lithium and fluorine. This polarity influences the compound's properties, such as its solubility and reactivity.
Conclusion: A Powerful Visualization Tool
Lewis dot diagrams, while simple in their depiction, serve as a crucial tool for visualizing the fundamental process of ionic bonding. By understanding the electron transfer and the achievement of stable octets, we can grasp the essence of the strong ionic bond formed between lithium and fluorine. While more complex models are required for in-depth analysis, the Lewis dot diagram provides a foundational understanding of this essential concept in chemistry. This understanding forms a solid base for exploring further complexities within ionic bonding and the properties of ionic compounds.
Latest Posts
Latest Posts
-
Equation Relating Electric Field And Voltage
Mar 31, 2025
-
Does A Plant Cell Have Dna
Mar 31, 2025
-
Concept Map Sympathetic And Parasympathetic Responses
Mar 31, 2025
-
What Are Coefficients In A Chemical Equation
Mar 31, 2025
-
Use Inverse Matrix To Solve Linear System
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Lewis Dot Diagram For Ionic Bonding Between Li And F . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
