What Are Coefficients In A Chemical Equation
Muz Play
Mar 31, 2025 · 6 min read
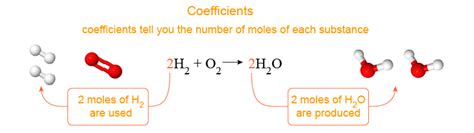
Table of Contents
What are Coefficients in a Chemical Equation? A Deep Dive into Balancing Equations
Chemical equations are the shorthand language of chemistry, representing the transformation of reactants into products. Understanding these equations is crucial for predicting the outcome of chemical reactions and performing stoichiometric calculations. A key element in understanding chemical equations is grasping the significance of coefficients. This article will delve deep into the meaning and importance of coefficients in chemical equations, exploring their role in balancing equations and their applications in various chemical contexts.
Understanding Chemical Equations
Before we dissect coefficients, let's establish a fundamental understanding of chemical equations. A chemical equation uses chemical formulas to represent the substances involved in a chemical reaction. For example:
2H₂ + O₂ → 2H₂O
This equation describes the reaction between hydrogen gas (H₂) and oxygen gas (O₂) to produce water (H₂O). The arrow (→) indicates the direction of the reaction, from reactants (on the left) to products (on the right).
What are Coefficients?
Coefficients are the numerical values written in front of the chemical formulas in a balanced chemical equation. They indicate the relative number of moles of each substance involved in the reaction. In the example above, the coefficients are 2, 1, and 2. These coefficients are crucial because they:
-
Represent the relative number of moles: The coefficient '2' in front of H₂ signifies that two moles of hydrogen gas react. Similarly, one mole of oxygen gas reacts (implied '1' coefficient) and two moles of water are produced.
-
Maintain the law of conservation of mass: Chemical reactions must obey the law of conservation of mass, stating that matter cannot be created or destroyed. Coefficients ensure that the number of atoms of each element is the same on both sides of the equation. This is known as a balanced chemical equation.
-
Provide mole ratios: Coefficients provide crucial mole ratios between reactants and products. This information is essential for stoichiometric calculations—determining the amounts of reactants needed or products formed in a reaction.
Why are Coefficients Important?
Coefficients are not merely numbers; they are essential components of a chemical equation, influencing several aspects:
-
Accuracy of Calculations: Incorrect coefficients lead to inaccurate stoichiometric calculations. These errors can have significant consequences, especially in industrial processes or laboratory experiments where precise measurements are crucial.
-
Predicting Reaction Outcomes: Coefficients allow chemists to predict the amounts of products formed from given amounts of reactants. This predictive power is invaluable in various applications, from designing chemical syntheses to analyzing environmental pollution.
-
Understanding Reaction Mechanisms: While coefficients don't directly reveal the reaction mechanism (the step-by-step process of a reaction), they provide crucial quantitative data that can help researchers infer mechanistic details.
Balancing Chemical Equations: The Role of Coefficients
Balancing a chemical equation is the process of adjusting coefficients to ensure that the number of atoms of each element is equal on both sides of the equation. This process is governed by the law of conservation of mass.
Steps to Balancing Chemical Equations:
-
Write the unbalanced equation: Write the chemical formulas of the reactants and products with an arrow separating them.
-
Count the atoms: Count the number of atoms of each element on both sides of the equation.
-
Adjust Coefficients: Systematically adjust the coefficients to balance the number of atoms of each element. Start with elements that appear only once on each side. Avoid changing the subscripts within the chemical formulas.
-
Verify the balance: After adjusting the coefficients, verify that the number of atoms of each element is the same on both sides of the equation.
Example: Balancing the combustion of methane
The unbalanced equation for the combustion of methane (CH₄) is:
CH₄ + O₂ → CO₂ + H₂O
Following the steps above:
-
Count the atoms: Reactants: 1C, 4H, 2O; Products: 1C, 2H, 3O
-
Adjust Coefficients: To balance the hydrogen, add a coefficient of 2 to H₂O:
CH₄ + O₂ → CO₂ + 2H₂O
Now we have: Reactants: 1C, 4H, 2O; Products: 1C, 4H, 4O
- Balance Oxygen: To balance oxygen, add a coefficient of 2 to O₂:
CH₄ + 2O₂ → CO₂ + 2H₂O
Now the equation is balanced: Reactants: 1C, 4H, 4O; Products: 1C, 4H, 4O
Beyond Simple Balancing: Complex Scenarios and Considerations
While balancing simple equations is relatively straightforward, balancing more complex equations can be challenging and may require iterative adjustments of coefficients. Here are some scenarios requiring more sophisticated techniques:
-
Redox Reactions: Balancing redox reactions (reactions involving electron transfer) requires additional steps, often involving half-reactions and the determination of oxidation states.
-
Reactions with Polyatomic Ions: When dealing with polyatomic ions (like sulfate, SO₄²⁻), it's helpful to treat the ion as a single unit when balancing. Adjust the coefficient for the entire ion rather than individual atoms within the ion.
-
Fractional Coefficients: Sometimes, balancing may require using fractional coefficients. While these are mathematically acceptable, they are often converted to whole numbers by multiplying all coefficients by a common denominator.
Practical Applications of Coefficients in Chemistry
The importance of coefficients extends beyond the theoretical realm of balancing equations. They are integral to numerous practical applications in chemistry, including:
-
Stoichiometry: Coefficients are fundamental to stoichiometric calculations, allowing us to determine the amount of reactants needed or products formed. For instance, in the methane combustion example, the coefficients indicate that one mole of methane reacts with two moles of oxygen to produce one mole of carbon dioxide and two moles of water.
-
Limiting Reactant Determination: Coefficients are essential for identifying the limiting reactant in a chemical reaction. The limiting reactant is the reactant that is completely consumed first, thus limiting the amount of product formed.
-
Percent Yield Calculations: Coefficients are crucial for calculating the theoretical yield of a reaction (the maximum amount of product that can be formed) and the percent yield (the actual yield compared to the theoretical yield).
-
Industrial Chemistry: In industrial settings, precise stoichiometric calculations based on coefficients are critical for optimizing reaction efficiency, minimizing waste, and maximizing product yield.
-
Environmental Chemistry: Understanding the stoichiometry of reactions, guided by coefficients, helps in assessing the impact of pollutants and developing strategies for environmental remediation.
Conclusion: Coefficients – The Unsung Heroes of Chemical Equations
In conclusion, coefficients are not merely numerical prefixes in chemical equations; they are vital components that underpin our understanding of chemical reactions. Their role in balancing equations, ensuring adherence to the law of conservation of mass, and facilitating stoichiometric calculations is indispensable. Mastering the use and interpretation of coefficients is a cornerstone of proficiency in chemistry, empowering us to accurately predict reaction outcomes, optimize processes, and solve various chemical problems. Understanding coefficients is a crucial step towards a deeper appreciation of the elegance and power of chemical equations.
Latest Posts
Latest Posts
-
How To Calculate Standard Free Energy Change
Apr 01, 2025
-
Root Mean Square Velocity Of Gas
Apr 01, 2025
-
Where Is The Respiratory Center Located In The Brain
Apr 01, 2025
-
What Is The Difference Between Cellular Respiration And Fermentation
Apr 01, 2025
-
What Are Two Functional Groups Found In Amino Acids
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Are Coefficients In A Chemical Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
