The Si Unit Of Energy Is The:
Muz Play
Mar 28, 2025 · 6 min read
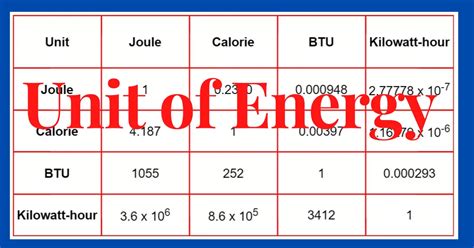
Table of Contents
The SI Unit of Energy Is the: Joule (J) – A Deep Dive into Energy Measurement
The fundamental question, "What is the SI unit of energy?", has a straightforward answer: the joule (J). However, understanding the joule and its implications goes far beyond a simple definition. This comprehensive guide will explore the joule, its relationship to other energy units, its applications across various scientific disciplines, and the importance of accurate energy measurement in our technologically advanced world.
Understanding the Joule: A Definition and Its Significance
The joule, named after the 19th-century physicist James Prescott Joule, is the International System of Units (SI) unit of energy. It's a measure of the work done or energy transferred when a force of one newton (N) acts over a distance of one meter (m). This seemingly simple definition underlies a vast array of physical phenomena, from the kinetic energy of a moving object to the potential energy stored in a stretched spring or the thermal energy contained within a substance.
Mathematically, the joule can be expressed as:
1 J = 1 N⋅m = 1 kg⋅m²/s²
This equation reveals the fundamental units that constitute the joule: kilograms (kg) for mass, meters (m) for distance, and seconds (s) for time. This interconnectedness highlights the underlying principles of mechanics and energy conservation.
The Joule's Importance Across Disciplines
The joule's significance transcends the realm of pure physics. Its application spans diverse fields:
-
Physics: Calculating kinetic energy (½mv²), potential energy (mgh), work done by a force, and understanding energy transformations in various systems.
-
Chemistry: Measuring the enthalpy changes in chemical reactions (e.g., heat released or absorbed), determining bond energies, and analyzing thermodynamic processes.
-
Engineering: Designing energy-efficient systems, analyzing power consumption in electrical circuits, and evaluating the performance of mechanical devices.
-
Medicine: Measuring the energy delivered by medical devices, like lasers used in surgery or radiation therapy.
-
Environmental Science: Assessing energy consumption and its impact on the environment, evaluating renewable energy sources, and understanding energy flows in ecosystems.
The Joule and Other Energy Units: Conversions and Equivalencies
While the joule is the standard SI unit, other energy units are commonly used depending on the context. Understanding the conversions between these units is crucial for clear communication and accurate calculations.
1. The Calorie (cal) and Kilocalorie (kcal):
The calorie, a non-SI unit, is often used in the context of food and nutrition. One calorie is defined as the amount of energy required to raise the temperature of one gram of water by one degree Celsius. The kilocalorie (kcal), also known as the "Calorie" (with a capital C) in dietary contexts, is equal to 1000 calories.
Conversion: 1 cal ≈ 4.184 J; 1 kcal ≈ 4184 J
2. The Kilowatt-hour (kWh):
The kilowatt-hour (kWh) is a unit of energy commonly used for electricity billing. It represents the energy consumed by a 1-kilowatt (kW) device operating for one hour.
Conversion: 1 kWh = 3.6 × 10⁶ J
3. The Electronvolt (eV):
The electronvolt (eV) is a unit of energy used in atomic and nuclear physics. It's defined as the energy gained by an electron when it's accelerated through a potential difference of one volt.
Conversion: 1 eV ≈ 1.602 × 10⁻¹⁹ J
4. The British Thermal Unit (BTU):
The British thermal unit (BTU) is a unit of energy commonly used in the United States for heating and cooling systems. It represents the amount of heat required to raise the temperature of one pound of water by one degree Fahrenheit.
Conversion: 1 BTU ≈ 1055 J
Accurate Energy Measurement: Techniques and Instruments
Precise energy measurement is fundamental to scientific research, engineering design, and many industrial processes. Various techniques and instruments are employed depending on the type and magnitude of energy being measured.
1. Calorimetry:
Calorimetry involves measuring the heat released or absorbed during a chemical or physical process. Different types of calorimeters, such as bomb calorimeters and differential scanning calorimeters (DSC), are used depending on the specific application.
2. Power Meters:
Power meters measure the rate of energy transfer (power) and can be used to determine the total energy consumed over a given period. These are commonly used to measure electricity consumption in homes and businesses.
3. Spectrometers:
Spectrometers measure the energy of electromagnetic radiation (light), allowing scientists to analyze the energy levels of atoms and molecules.
4. Thermometry:
Thermometry, the measurement of temperature, is indirectly related to energy measurement. Changes in temperature are often associated with changes in energy.
The Joule and Energy Conservation: A Fundamental Principle
The concept of the joule is intrinsically linked to the principle of energy conservation. This fundamental principle of physics states that energy cannot be created or destroyed, only transformed from one form to another. The total energy within a closed system remains constant. The joule, as the universal unit of energy, provides a consistent framework for tracking these transformations and verifying the validity of energy conservation in various processes.
For instance, when a ball is dropped, its potential energy is converted into kinetic energy. By accurately measuring the initial potential energy and the final kinetic energy using the joule as the unit, we can demonstrate the conservation of energy. Similarly, in chemical reactions, the change in enthalpy (heat) can be measured in joules, illustrating the transformation of chemical energy into thermal energy.
Applications of Energy Measurement in Everyday Life
The accurate measurement of energy isn't confined to scientific laboratories; it plays a significant role in our daily lives:
-
Electricity Bills: Our electricity consumption is measured in kilowatt-hours (kWh), which are directly related to joules. Understanding energy consumption patterns allows for more efficient use of energy resources and reduced costs.
-
Fuel Efficiency: The energy content of fuels, like gasoline, is expressed in joules per liter or BTU per gallon. Understanding this value is crucial for evaluating fuel efficiency in vehicles and choosing fuel-efficient options.
-
Dietary Needs: The energy content of food is measured in kilocalories (kcal) which are crucial for managing weight and maintaining healthy lifestyles.
-
Renewable Energy Sources: Evaluating the energy output of solar panels, wind turbines, and other renewable energy technologies relies on precise energy measurements in joules. This allows for optimizing their design and maximizing their efficiency.
The Future of Energy Measurement and the Joule
As our technological capabilities advance, the precision and scope of energy measurement are continuously improving. New techniques and instruments are being developed to address the challenges of measuring energy in increasingly complex systems, from nanoscale devices to large-scale energy grids.
The joule, as the foundation of energy measurement, will undoubtedly remain central to these advancements. Its clear definition and universal applicability make it the ideal unit for expressing and quantifying energy in a wide range of applications, ensuring accurate calculations, efficient energy management, and a deeper understanding of the universe's fundamental forces. The ongoing development and refinement of energy measurement techniques based on the joule will continue to contribute significantly to scientific progress, technological innovation, and environmental sustainability. The joule, therefore, is not merely a unit; it’s a cornerstone of our understanding and utilization of one of the universe’s most fundamental quantities.
Latest Posts
Latest Posts
-
What Happens To An Animal Cell In A Isotonic Solution
Mar 31, 2025
-
Atom That Has Gained Or Lost Electrons
Mar 31, 2025
-
Cuantas Onzas Tiene Un Cuarto De Galon
Mar 31, 2025
-
Work Done By A Varying Force
Mar 31, 2025
-
The Rna Components Of Ribosomes Are Synthesized In The
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about The Si Unit Of Energy Is The: . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
