What Happens To An Animal Cell In A Isotonic Solution
Muz Play
Mar 31, 2025 · 5 min read
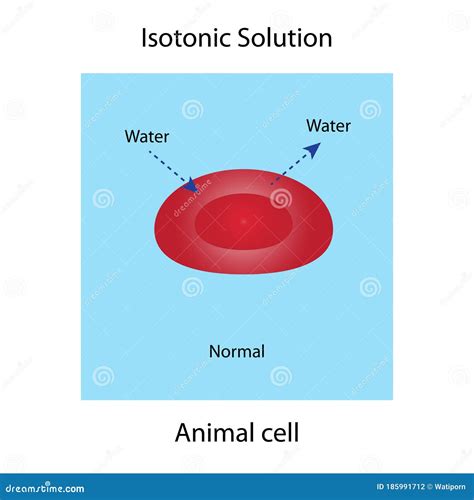
Table of Contents
What Happens to an Animal Cell in an Isotonic Solution? A Deep Dive into Osmosis and Cellular Equilibrium
Understanding how cells behave in different environments is crucial to comprehending basic biology. One key concept is the interaction between a cell and its surrounding solution's tonicity – specifically, what happens when an animal cell is placed in an isotonic solution. This article delves deep into the process of osmosis in animal cells, exploring the impact of isotonic solutions and contrasting it with hypertonic and hypotonic environments. We'll also examine the implications for cell function and survival.
Understanding Osmosis: The Driving Force
Osmosis is the passive movement of water molecules across a selectively permeable membrane. This movement is driven by a difference in water potential between two solutions. Water potential is essentially the tendency of water to move from an area of high concentration to an area of low concentration. This movement continues until equilibrium is reached, meaning the water potential is equal on both sides of the membrane.
The presence of solutes (dissolved substances) in water lowers its water potential. The more solutes present, the lower the water potential. This is a crucial factor in understanding how cells respond to different solutions.
Selectively Permeable Membranes: The Gatekeepers
Animal cells are surrounded by a plasma membrane, a selectively permeable barrier. This membrane allows some substances to pass through freely while restricting others. Water molecules, being small and uncharged, can pass relatively easily through the plasma membrane via osmosis. Larger molecules or charged ions require specific transport mechanisms to cross the membrane.
Isotonic Solutions: The Goldilocks Zone for Animal Cells
An isotonic solution is one where the concentration of solutes is equal both inside and outside the cell. This means the water potential is also equal on both sides of the plasma membrane. This equilibrium is vital for maintaining cell structure and function.
What Happens in an Isotonic Environment?
When an animal cell is placed in an isotonic solution:
- No net movement of water: Because the water potential is the same inside and outside the cell, there's no driving force for water to move across the membrane in either direction. Water molecules still move across the membrane, but the movement is equal in both directions, resulting in no net change in cell volume.
- Stable cell volume: The cell maintains its normal shape and size. It neither swells nor shrinks.
- Optimal cellular function: The stable internal environment allows for optimal cellular processes to occur. Transport mechanisms, enzyme activity, and other cellular functions operate efficiently.
Contrast with Hypertonic and Hypotonic Solutions
Understanding what happens in an isotonic environment is best done by comparing it to what happens in hypertonic and hypotonic solutions.
Hypertonic Solutions: Water Loss and Cell Shrinkage
A hypertonic solution has a higher concentration of solutes than the inside of the cell. This means the water potential outside the cell is lower than inside the cell. As a result:
- Water moves out of the cell: Water passively moves from the area of higher water potential (inside the cell) to the area of lower water potential (outside the cell) to try and equalize the concentrations.
- Cell shrinkage (crenation): The loss of water causes the cell to shrink and become crenated. The cell membrane pulls away from the cell wall (if present) and the cell's internal contents become concentrated.
- Disruption of cellular processes: The changes in cell volume and internal concentration can disrupt cellular processes, potentially leading to cell death if the water loss is severe.
Hypotonic Solutions: Water Gain and Cell Lysis
A hypotonic solution has a lower concentration of solutes than the inside of the cell. This means the water potential outside the cell is higher than inside the cell. Consequently:
- Water moves into the cell: Water passively moves from the area of higher water potential (outside the cell) to the area of lower water potential (inside the cell).
- Cell swelling: The influx of water causes the cell to swell.
- Cell lysis (bursting): Animal cells lack a rigid cell wall, so excessive water intake can lead to the cell membrane rupturing, causing the cell to burst and die. This process is known as lysis. Plant cells, with their cell walls, can withstand some swelling and become turgid, but excessive water can still damage them.
The Importance of Maintaining Isotonicity
Maintaining an isotonic internal environment is crucial for the survival and proper functioning of animal cells. The body has various mechanisms to regulate the concentration of solutes in the extracellular fluid, ensuring that cells are exposed to an isotonic environment. For example, the kidneys play a vital role in maintaining proper electrolyte balance, influencing the tonicity of the blood and surrounding tissues.
Practical Applications and Further Exploration
The principles of osmosis and tonicity have numerous practical applications:
- Medicine: Intravenous (IV) fluids are carefully formulated to be isotonic with blood plasma to avoid damaging red blood cells. Hypotonic or hypertonic IV solutions can have serious consequences.
- Agriculture: Understanding osmosis helps farmers manage irrigation and fertilization practices to optimize plant growth.
- Food preservation: Osmosis is involved in methods like pickling and curing, where solutes are added to create a hypertonic environment that inhibits microbial growth.
Further Research Areas:
- The role of aquaporins (water channels) in facilitating water movement across cell membranes.
- The complexities of osmosis in multicellular organisms, where different cell types may have different osmotic requirements.
- The development of novel techniques for manipulating cell tonicity for therapeutic purposes.
Conclusion: A Balanced Act for Cellular Survival
The behavior of an animal cell in an isotonic solution highlights the fundamental importance of osmosis and maintaining cellular equilibrium. The isotonic environment ensures the cell neither gains nor loses water excessively, allowing for optimal function and survival. Understanding the contrast with hypertonic and hypotonic solutions illuminates the delicate balance necessary for cellular health, a balance with far-reaching implications across various biological systems and applications. Further research continues to unravel the intricate details of these processes, pushing the boundaries of our understanding of cellular life.
Latest Posts
Latest Posts
-
What Is In The Atmosphere Of Jupiter
Apr 01, 2025
-
What Does A Negative Reduction Potential Mean
Apr 01, 2025
-
How To Calculate Standard Free Energy Change
Apr 01, 2025
-
Root Mean Square Velocity Of Gas
Apr 01, 2025
-
Where Is The Respiratory Center Located In The Brain
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Happens To An Animal Cell In A Isotonic Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
