What Does A Negative Reduction Potential Mean
Muz Play
Apr 01, 2025 · 6 min read
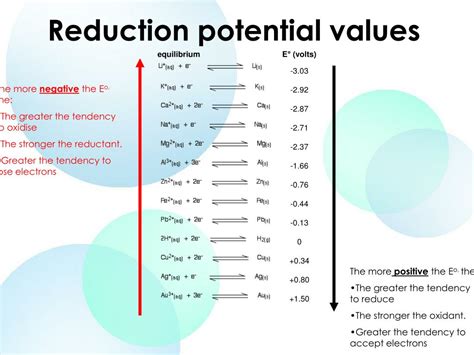
Table of Contents
What Does a Negative Reduction Potential Mean?
Understanding reduction potential is crucial in various fields, from chemistry and electrochemistry to biology and environmental science. This comprehensive guide delves into the meaning of a negative reduction potential, exploring its implications and applications. We'll unravel the complexities, providing clear explanations and real-world examples to solidify your understanding.
Understanding Reduction Potential: The Basics
Before diving into negative reduction potentials, let's establish a firm foundation. Reduction potential, often expressed as E⁰ (standard reduction potential), quantifies a substance's tendency to gain electrons and undergo reduction. Reduction is a fundamental chemical process where a species gains electrons, decreasing its oxidation state. The reduction potential is measured in volts (V) relative to a standard hydrogen electrode (SHE), which is arbitrarily assigned a potential of 0 V.
A higher positive reduction potential indicates a stronger tendency for a substance to be reduced (i.e., it readily accepts electrons). Conversely, a lower reduction potential (including negative values) suggests a weaker tendency for reduction. This doesn't mean it won't be reduced; it simply requires a stronger reducing agent to facilitate the process.
The reduction potential is intrinsically linked to the oxidation potential. Oxidation, the opposite of reduction, involves the loss of electrons. The oxidation potential is simply the negative of the reduction potential. Therefore, a highly positive reduction potential corresponds to a highly negative oxidation potential, and vice versa.
Decoding Negative Reduction Potentials
A negative reduction potential signifies that a substance is a relatively strong reducing agent. This means it readily donates electrons to other substances, causing those other substances to be reduced. The more negative the reduction potential, the stronger the reducing power of the substance.
Think of it like this: electrons are like a ball. A substance with a positive reduction potential acts like a person who eagerly catches the ball (accepts electrons). A substance with a negative reduction potential acts like a person who readily throws the ball (donates electrons). The more negative the potential, the more vigorously they throw the ball.
Key takeaway: A negative reduction potential doesn't mean the substance cannot be reduced. It simply implies that it requires a stronger oxidizing agent (something that readily accepts electrons) to force the reduction process.
The Significance of the Standard Hydrogen Electrode (SHE)
The SHE serves as the reference point for all reduction potentials. Its potential is defined as 0 V under standard conditions (25°C, 1 atm pressure, 1 M concentration of ions). All other reduction potentials are measured relative to the SHE. This standardization allows for consistent and comparable measurements across various electrochemical reactions.
The SHE, however, isn't always practical for everyday measurements. Because of this, other reference electrodes, such as the saturated calomel electrode (SCE) and silver/silver chloride electrode (Ag/AgCl), are frequently used. Their potentials are well-established relative to the SHE, allowing for easy conversion between different reference electrodes.
Applications of Negative Reduction Potentials
Negative reduction potentials are pivotal in several areas:
1. Electrochemistry and Battery Technology
Many batteries rely on redox reactions involving substances with significantly different reduction potentials. The negative electrode (anode) typically contains a material with a very negative reduction potential, acting as the electron donor. This electron flow drives the electrical current in the battery. Lithium-ion batteries, for example, utilize lithium metal with its extremely negative reduction potential as the anode material.
2. Corrosion Prevention
Understanding reduction potentials is essential in preventing corrosion. Materials with negative reduction potentials are more susceptible to oxidation (corrosion) when in contact with substances with more positive reduction potentials. This knowledge informs the selection of materials for specific environments and the implementation of corrosion protection strategies, such as cathodic protection.
3. Environmental Remediation
Substances with negative reduction potentials play a crucial role in environmental remediation techniques. For instance, certain metals can be removed from contaminated water by employing reduction processes using strong reducing agents with negative reduction potentials. These reducing agents facilitate the reduction and precipitation of the metal ions, effectively removing them from the water.
4. Biological Systems
In biological systems, electron transfer processes are central to various metabolic pathways. Many enzymes involved in respiration and photosynthesis utilize redox reactions involving molecules with negative reduction potentials. These reactions facilitate the transfer of electrons through electron transport chains, generating energy for cellular processes. For example, the reduction potential of NADH (nicotinamide adenine dinucleotide) plays a key role in cellular respiration.
Factors Affecting Reduction Potential
Several factors can influence the reduction potential of a substance:
-
Concentration: Changes in the concentration of reactants and products affect the reduction potential via the Nernst equation. This equation mathematically relates the standard reduction potential to the actual reduction potential under non-standard conditions.
-
Temperature: Temperature alterations can impact the reduction potential, albeit usually to a lesser extent than concentration changes.
-
pH: The pH of the solution significantly affects the reduction potential, particularly for reactions involving protons (H⁺) or hydroxide ions (OH⁻).
-
Presence of complexing agents: Ligands that form complexes with metal ions can alter the reduction potential of the metal ion.
Real-World Examples of Negative Reduction Potentials
Let's examine some real-world examples to solidify our understanding:
-
Lithium (Li): Lithium metal has an extremely negative standard reduction potential (-3.04 V). This makes it a powerful reducing agent and explains its use in high-energy-density batteries.
-
Sodium (Na): Sodium also possesses a highly negative reduction potential (-2.71 V), contributing to its role in various electrochemical applications.
-
Magnesium (Mg): Magnesium's negative reduction potential (-2.37 V) makes it a sacrificial anode in cathodic protection systems, preventing corrosion of other metals.
-
Zinc (Zn): Zinc's negative reduction potential (-0.76 V) contributes to its use in galvanic cells (batteries) and as a coating to protect steel from corrosion.
-
Iron (Fe): Iron has a relatively less negative reduction potential (-0.44 V), making it prone to corrosion in certain environments.
It's important to note that these are standard reduction potentials. The actual reduction potential will vary depending on the specific conditions.
Conclusion
Understanding negative reduction potentials is fundamental to various scientific and technological disciplines. A negative reduction potential signifies that a substance is a strong reducing agent, readily donating electrons to other species. This property finds applications in battery technology, corrosion prevention, environmental remediation, and biological processes. By grasping the concepts of reduction potential, its influencing factors, and real-world applications, one gains a deeper appreciation for the intricate world of electrochemistry and its pervasive impact. Remember that while a negative reduction potential indicates a strong reducing agent, it doesn't preclude the substance from undergoing reduction; it simply requires a more potent oxidizing agent to drive the process. This nuanced understanding is critical for diverse applications, enabling informed decision-making in various fields.
Latest Posts
Latest Posts
-
The Final Electron Acceptor Of Cellular Respiration Is
Apr 02, 2025
-
Monomers Are Connected In What Type Of Reaction
Apr 02, 2025
-
Find The Equation Of The Vertical Line
Apr 02, 2025
-
Electron Configuration For Copper And Chromium
Apr 02, 2025
-
Confidence Interval Calculator With Two Samples
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Does A Negative Reduction Potential Mean . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
