What Type Of Energy Is Created By Breaking The Bonds
Muz Play
Mar 22, 2025 · 6 min read
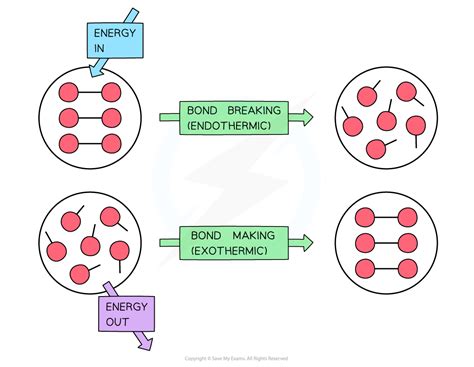
Table of Contents
What Type of Energy is Created by Breaking Bonds?
The breaking of chemical bonds is a fundamental process in chemistry and physics, releasing energy in various forms. Understanding the nature of this energy release is crucial across numerous scientific disciplines, from understanding basic chemical reactions to developing advanced energy technologies. This article explores the types of energy created when bonds break, the underlying mechanisms, and examples across different contexts.
The Nature of Chemical Bonds and Energy
Before diving into the energy released upon bond breakage, it's essential to understand what chemical bonds are and how they store energy. Chemical bonds are the forces that hold atoms together in molecules or crystals. These bonds arise from the electrostatic interactions between electrons and nuclei of the atoms involved. The stability of a bond is determined by the balance between attractive and repulsive forces. When atoms bond, they achieve a lower energy state compared to their isolated, unbound state. This difference in energy is the bond energy.
Types of Chemical Bonds
Different types of chemical bonds store energy in varying amounts. The most common types include:
-
Covalent Bonds: These bonds involve the sharing of electrons between atoms. The stronger the electron sharing, the stronger and more stable the bond, requiring a greater amount of energy to break. Examples abound in organic molecules like those forming proteins and DNA.
-
Ionic Bonds: These bonds involve the transfer of electrons from one atom to another, creating ions with opposite charges that attract each other. The strength of ionic bonds is heavily influenced by the charges of the ions and the distance between them. Salt crystals (NaCl) are a classic example.
-
Metallic Bonds: These bonds are found in metals and involve a "sea" of delocalized electrons shared among a lattice of metal atoms. The strength of metallic bonds depends on the number of valence electrons and the size of the atoms.
-
Hydrogen Bonds: These are weaker than covalent, ionic, and metallic bonds, resulting from the attraction between a hydrogen atom bonded to a highly electronegative atom (like oxygen or nitrogen) and another electronegative atom. Although individually weak, collectively, they play a vital role in the structure and function of biomolecules like proteins and DNA.
-
Van der Waals Forces: These are the weakest type of intermolecular force, resulting from temporary fluctuations in electron distribution around atoms or molecules. While individually weak, collectively they contribute significantly to the properties of many substances.
Energy Released Upon Bond Breaking: A Closer Look
When a chemical bond breaks, the energy stored within that bond is released. This energy can manifest in several forms:
1. Heat (Thermal Energy):
This is the most common form of energy released when bonds break. The released energy increases the kinetic energy of the surrounding molecules, leading to an increase in temperature. Many exothermic reactions, such as combustion, release significant amounts of heat as bonds break and new ones form. The burning of wood or natural gas are prime examples where the breaking of chemical bonds leads to a significant temperature increase.
2. Light (Radiant Energy):
Certain chemical reactions release energy in the form of light. This often happens when the energy released excites electrons to higher energy levels, and as these electrons return to lower energy levels, they emit photons of light. Chemiluminescence, the production of light as a result of a chemical reaction, is a classic illustration. Fireflies and glow sticks exemplify chemiluminescence, generating light as chemical bonds are broken and reformed.
3. Electrical Energy:
Breaking bonds can lead to the generation of electrical energy, particularly in electrochemical reactions occurring in batteries or fuel cells. In these systems, the breaking of bonds results in the movement of electrons, creating an electric current. The energy stored in the chemical bonds is transformed into electrical energy that can power various devices. This is how batteries function: chemical reactions break down substances to liberate electrons that flow through an external circuit.
4. Mechanical Energy:
The breaking of bonds can also lead to the release of mechanical energy. For instance, the rapid expansion of gases due to bond breakage in explosives creates a powerful mechanical force, producing work such as blasting or propulsion. The decomposition of energetic materials like gunpowder or nitroglycerin exemplifies this – the rapid bond breaking leads to an explosion, converting chemical energy into kinetic and mechanical energy.
5. Sound Energy:
In some cases, the breaking of bonds can produce sound energy. The rapid expansion of gases or the sudden movement of molecules due to bond breakage can create pressure waves that propagate as sound. The cracking of a twig, an explosion, or even the snapping of certain materials involve bond breakage leading to the generation of sound waves.
Examples Across Different Contexts
The release of energy from bond breaking is not limited to a few specific reactions; it’s a ubiquitous phenomenon found in various contexts:
1. Combustion:
Combustion, the rapid reaction of a substance with oxygen, involves the breaking of bonds in the fuel and oxygen molecules. The energy released is mainly in the form of heat and light, as exemplified by burning wood, gas, or other fuels. The formation of new, stronger bonds in carbon dioxide and water molecules contributes to the overall energy release.
2. Respiration:
Cellular respiration, the process by which living organisms obtain energy from food, involves breaking down complex organic molecules such as glucose. The breaking of the bonds in glucose releases energy, which is then used to drive various cellular processes. This energy is ultimately released as ATP (adenosine triphosphate), the cell's primary energy currency.
3. Photosynthesis:
While photosynthesis involves bond formation, it also involves bond breaking. The energy from sunlight is used to break the bonds in water molecules, releasing oxygen and providing the energy to drive the synthesis of glucose. The initial energy input is needed to initiate the bond-breaking process that is essential for carbohydrate synthesis.
4. Explosions:
Explosions involve the extremely rapid breaking of bonds in unstable molecules. This leads to a rapid expansion of gases, producing a large amount of mechanical energy and often sound and light as well. The shockwave produced from an explosion is a direct consequence of this rapid energy release due to bond breakage. Dynamite and other explosives rely on this principle.
5. Nuclear Reactions:
While not strictly chemical bond breaking, nuclear reactions involve the breaking of bonds within the atomic nucleus. This releases enormous amounts of energy, far exceeding that released in chemical reactions. Nuclear fission and fusion are prime examples, releasing energy in the form of heat, light, and kinetic energy of the resulting particles.
Conclusion
The breaking of chemical bonds is a fundamental process that underpins a vast array of phenomena, releasing energy in various forms—heat, light, electricity, mechanical energy, and sound. Understanding the nature of this energy release is crucial in fields ranging from chemistry and physics to biology and engineering. From combustion engines to biological processes, the principles of bond breaking and energy transformation remain essential concepts in our quest to understand and harness energy from the natural world. Further research continually expands our understanding of these processes, leading to advancements in energy production, materials science, and other vital technologies.
Latest Posts
Latest Posts
-
Bohr Diagrams For The First 20 Elements
Mar 22, 2025
-
What Is The Property Of Bases
Mar 22, 2025
-
Is A Cow A First Level Consumer
Mar 22, 2025
-
What Is The Heat Of Vaporization Of Water
Mar 22, 2025
-
What Is Electronic Configuration Of Carbon
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Energy Is Created By Breaking The Bonds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
