What Is Electronic Configuration Of Carbon
Muz Play
Mar 22, 2025 · 6 min read
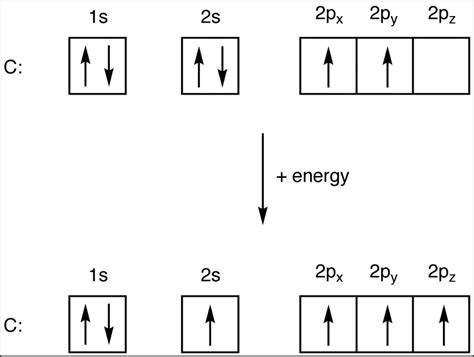
Table of Contents
What is the Electronic Configuration of Carbon? A Deep Dive into Atomic Structure and Chemical Bonding
Carbon, the cornerstone of organic chemistry and life itself, boasts a deceptively simple yet profoundly influential electronic configuration. Understanding this configuration is crucial to grasping carbon's remarkable versatility in forming a vast array of molecules, from simple hydrocarbons to complex biomolecules like DNA. This article will delve deep into the electronic configuration of carbon, exploring its implications for chemical bonding, molecular structure, and its significance in the natural world.
Understanding Electronic Configuration: The Basics
Before we dive into carbon's specific electronic configuration, let's establish a fundamental understanding of what electronic configuration represents. An atom's electronic configuration describes how electrons are distributed among its various energy levels and sublevels. These energy levels are often depicted as shells or orbitals, with electrons occupying orbitals according to specific rules dictated by quantum mechanics.
The arrangement of electrons determines an atom's chemical properties and reactivity. Electrons in the outermost shell, known as valence electrons, are particularly important as they participate directly in chemical bonding with other atoms.
The electronic configuration is typically represented using a notation system that specifies the principal quantum number (n), the orbital type (s, p, d, f), and the number of electrons in each orbital. For example, 1s² denotes two electrons in the 1s orbital (principal quantum number 1, s orbital).
The Electronic Configuration of Carbon (C): 1s²2s²2p²
Carbon, with an atomic number of 6, possesses six electrons. Its electronic configuration is concisely written as 1s²2s²2p². Let's break this down:
-
1s²: Two electrons occupy the lowest energy level (n=1), specifically the 1s orbital. The 's' orbital is spherical and can hold a maximum of two electrons. These 1s electrons are closest to the nucleus and are strongly bound.
-
2s²: Two electrons occupy the 2s orbital (n=2). Similar to the 1s orbital, the 2s orbital is also spherical but larger and at a higher energy level than the 1s orbital.
-
2p²: Two electrons occupy the 2p orbitals (n=2). Unlike the s orbitals, the p orbitals are dumbbell-shaped and exist in three orientations (px, py, pz) along the x, y, and z axes. Each p orbital can accommodate a maximum of two electrons, and in carbon, only two of the three 2p orbitals are occupied.
This leaves carbon with four valence electrons – two in the 2s orbital and two in the 2p orbitals. This seemingly small number of valence electrons is the key to carbon's extraordinary ability to form a vast array of molecules.
Carbon's Valence Electrons and Chemical Bonding
The four valence electrons are responsible for carbon's remarkable bonding capabilities. Carbon readily forms covalent bonds by sharing its valence electrons with other atoms. This ability is crucial for forming the strong and stable bonds that underpin the structural diversity of organic molecules.
There are several types of chemical bonds carbon can form:
-
Single Bonds: Carbon can share one electron with another atom, forming a single covalent bond (represented by a single line – –). This is seen in molecules like methane (CH₄).
-
Double Bonds: Carbon can share two electrons with another atom, forming a double covalent bond (represented by a double line =). This is found in molecules like ethene (C₂H₄).
-
Triple Bonds: Carbon can share three electrons with another atom, forming a triple covalent bond (represented by a triple line ≡). This is seen in molecules like ethyne (C₂H₂).
The ability to form single, double, and triple bonds, along with the possibility of forming chains and rings, is what allows carbon to create the staggering diversity of organic compounds.
Hybridization: A Key to Carbon's Versatility
Carbon's electronic configuration allows for a phenomenon called hybridization, which significantly impacts its bonding behaviour and molecular geometry. Hybridization involves the mixing of atomic orbitals to create new hybrid orbitals with different shapes and energies.
The most common types of hybridization in carbon are:
-
sp³ Hybridization: This occurs when one 2s orbital and three 2p orbitals hybridize to form four equivalent sp³ hybrid orbitals. These orbitals are arranged tetrahedrally, with bond angles of approximately 109.5°. This hybridization is seen in methane (CH₄) and other alkanes.
-
sp² Hybridization: This occurs when one 2s orbital and two 2p orbitals hybridize to form three equivalent sp² hybrid orbitals. These orbitals are arranged in a trigonal planar geometry with bond angles of approximately 120°. One p orbital remains unhybridized and participates in the formation of a pi (π) bond. This hybridization is characteristic of alkenes like ethene (C₂H₄).
-
sp Hybridization: This involves the hybridization of one 2s orbital and one 2p orbital to form two equivalent sp hybrid orbitals. These orbitals are arranged linearly with a bond angle of 180°. Two p orbitals remain unhybridized and participate in the formation of two pi (π) bonds. This is seen in alkynes like ethyne (C₂H₂).
Hybridization is a crucial concept in understanding the molecular shapes and reactivity of organic molecules containing carbon.
Carbon's Significance in Organic Chemistry and Life
The unique electronic configuration of carbon, with its four valence electrons and its ability to form diverse bonds and undergo hybridization, makes it the central element of organic chemistry. Millions of organic compounds are based on carbon's ability to form stable chains, branches, and rings.
The importance of carbon extends far beyond organic chemistry, as it is fundamental to life itself. All living organisms are composed primarily of carbon-based molecules, including carbohydrates, lipids, proteins, and nucleic acids (DNA and RNA). These biomolecules perform various essential functions, from providing energy to storing genetic information.
Isotopes of Carbon and their Significance
Carbon has several naturally occurring isotopes, the most common being carbon-12 (¹²C) and carbon-13 (¹³C). These isotopes have the same number of protons and electrons but differ in the number of neutrons. Carbon-14 (¹⁴C), a radioactive isotope, is used in radiocarbon dating to determine the age of ancient organic materials. The different isotopes of carbon have slightly varying nuclear spins and masses, affecting their behaviour in certain analytical techniques such as nuclear magnetic resonance (NMR) spectroscopy.
Conclusion: The Enduring Importance of Carbon's Electronic Configuration
The electronic configuration of carbon, seemingly simple at first glance, underpins its incredible versatility and importance in the universe. Its four valence electrons, capacity for diverse bonding patterns, and the possibility of hybridization allow it to form the backbone of a vast array of molecules, from simple hydrocarbons to complex biomolecules that form the basis of life. Understanding this fundamental aspect of carbon's atomic structure is essential for comprehending its remarkable role in chemistry and biology. The continuing exploration and investigation of carbon-based compounds promise further insights into the multifaceted nature of this element and its critical contributions to the natural world.
Latest Posts
Latest Posts
-
Chemistry Is The Study Of Matter
Mar 23, 2025
-
How Does Sexual Reproduction Lead To Genetic Variation
Mar 23, 2025
-
Why Are Amino Acids Called Amino Acids
Mar 23, 2025
-
How Do You Find The Length Of A Vector
Mar 23, 2025
-
Lewis Structure Of Carbon Monoxide With Formal Charges
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is Electronic Configuration Of Carbon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
