Lewis Structure Of Carbon Monoxide With Formal Charges
Muz Play
Mar 23, 2025 · 5 min read
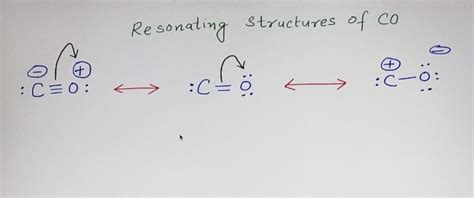
Table of Contents
Delving Deep into the Lewis Structure of Carbon Monoxide: A Comprehensive Guide
Carbon monoxide (CO), a simple yet fascinating molecule, presents a compelling case study in understanding Lewis structures and formal charges. Its seemingly straightforward composition belies a rich chemistry driven by its unique bonding characteristics. This article will provide a comprehensive exploration of the Lewis structure of carbon monoxide, including a detailed explanation of formal charges and their implications. We will also touch upon the molecule's resonance structures, bond order, and overall polarity. Understanding these concepts is crucial for grasping the reactivity and properties of this important molecule.
Understanding Lewis Structures: A Foundation
Before delving into the specifics of CO, let's revisit the fundamental principles of Lewis structures. These diagrams provide a visual representation of the valence electrons in a molecule, showing how atoms share electrons to achieve a stable octet (or duet for hydrogen). They are crucial for predicting molecular geometry and properties.
Key elements of a Lewis structure include:
- Valence electrons: The outermost electrons of an atom, which participate in bonding.
- Single, double, and triple bonds: Representations of electron pairs shared between atoms.
- Lone pairs: Pairs of electrons not involved in bonding.
- Formal charges: A calculation used to assess the distribution of electrons within a molecule. We’ll discuss this in detail later.
Constructing the Lewis Structure of Carbon Monoxide (CO)
To draw the Lewis structure of CO, we first determine the total number of valence electrons. Carbon has four valence electrons, and oxygen has six, giving a total of ten valence electrons.
-
Central Atom: Generally, the less electronegative atom is placed in the center. In this case, carbon is less electronegative than oxygen, and so it is the central atom.
-
Connecting Atoms: We connect the carbon and oxygen atoms with a single bond, using two valence electrons.
-
Satisfying the Octet Rule: We now distribute the remaining eight electrons to satisfy the octet rule for both atoms. Initially, we can add three lone pairs to oxygen, using six electrons. This leaves us with two electrons. However, both carbon and oxygen only have six electrons in their outer shell. This is where the concept of multiple bonds comes into play.
-
Multiple Bonds and Octet Rule Fulfillment: To fulfill the octet rule for both carbon and oxygen, we convert one of oxygen's lone pairs into a bonding pair, forming a double bond between carbon and oxygen. This uses four electrons, and now carbon and oxygen both have eight electrons in their outermost shell. However, this still isn’t the most accurate representation.
-
Triple Bond and Resonance: To achieve the lowest possible formal charges, we need to form a triple bond. By converting two lone pairs from the oxygen atom into bonding pairs, we form a triple bond (six shared electrons) between carbon and oxygen. This leaves one lone pair remaining on the oxygen atom and the carbon atom has no lone pairs. This structure is often written as: :C≡O:
-
Formal Charges Calculation and Evaluation: Before we definitively conclude that this is the correct Lewis structure, let's calculate formal charges to confirm its stability.
Formal Charges in Carbon Monoxide
The formal charge is a useful tool in evaluating the relative stability of different Lewis structures. It is calculated as follows:
Formal Charge = (Valence electrons) - (Non-bonding electrons) - (1/2 Bonding electrons)
Let's calculate the formal charges for each atom in the triple-bonded CO structure:
- Carbon: Formal Charge = 4 - 0 - (1/2 * 6) = +1
- Oxygen: Formal Charge = 6 - 2 - (1/2 * 6) = +1
Both the carbon and the oxygen atom have a formal charge of +1. While this doesn't necessarily mean it’s an incorrect structure, structures with lower formal charges are generally preferred. Since we have a triple bond, we now need to consider resonance to further refine our understanding of the CO bond.
Resonance Structures in Carbon Monoxide
Resonance describes the delocalization of electrons in a molecule, where multiple valid Lewis structures can be drawn that differ only in the placement of electrons. For CO, while the triple bond structure is the most common representation, we can consider other resonance forms, although they are significantly less significant in terms of overall contribution to the real structure. These resonance structures involve shifting electron density between the carbon and oxygen atoms, although none of them result in a neutral formal charge on both atoms.
Bond Order and Polarity in Carbon Monoxide
The bond order in CO is 3, indicating a strong triple bond. This high bond order contributes to the molecule's high bond energy and stability. However, despite the triple bond and equal number of electrons being shared, the molecule is polar. Oxygen is more electronegative than carbon. This means that the shared electrons are pulled more closely to the oxygen atom, creating a slight negative charge (δ-) on the oxygen and a slight positive charge (δ+) on the carbon.
Implications of the Lewis Structure and Formal Charges
The Lewis structure of CO, with its triple bond and formal charges, is crucial for understanding its chemical behavior:
-
Reactivity: The strong triple bond makes CO relatively unreactive compared to other molecules with double or single bonds. However, the slight polarity and presence of formal charges still allows it to participate in specific reactions.
-
Coordination Chemistry: The lone pair of electrons on the oxygen can coordinate to a metal center, forming metal carbonyl complexes – compounds of considerable importance in organometallic chemistry.
-
Toxicity: The strong binding affinity of CO to hemoglobin in the blood explains its toxicity. It prevents oxygen from binding, resulting in oxygen deprivation.
Conclusion
The Lewis structure of carbon monoxide, while seemingly simple at first glance, reveals a wealth of information about its bonding, polarity, and reactivity. Understanding the concepts of formal charges and resonance is crucial for correctly interpreting the electron distribution within the molecule. This knowledge enables us to predict and explain the diverse chemical properties of this important and widely studied molecule. Further investigation into advanced bonding theories can provide even deeper insight into the complexities of the carbon-oxygen bond. The apparent simplicity of this seemingly simple diatomic molecule masks a complex reality, one worthy of further study and exploration.
Latest Posts
Latest Posts
-
How To Do Inverse Laplace Transforms
Mar 25, 2025
-
Real World Application Of A Linear Equation In 2 Variables
Mar 25, 2025
-
Is Boil A Physical Or Chemical Change
Mar 25, 2025
-
How To Write All Real Numbers In Interval Notation
Mar 25, 2025
-
What Are Rows Called On The Periodic Table
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Lewis Structure Of Carbon Monoxide With Formal Charges . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
