Is Boil A Physical Or Chemical Change
Muz Play
Mar 25, 2025 · 4 min read
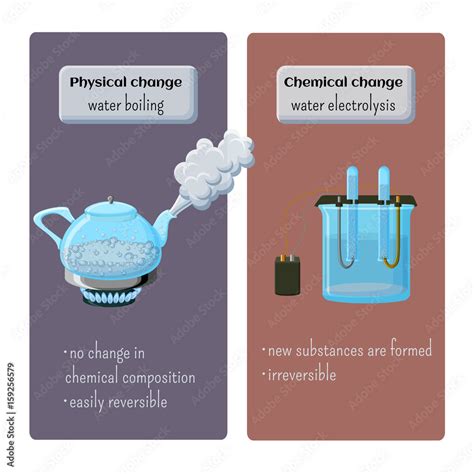
Table of Contents
Is Boiling a Physical or Chemical Change? A Deep Dive
The question of whether boiling is a physical or chemical change is a common one, often sparking debate among students and science enthusiasts alike. The answer, however, isn't a simple yes or no. Understanding the nuances requires a close examination of the definitions of physical and chemical changes and a detailed look at what happens at a molecular level when a liquid boils. This article will explore this fascinating topic thoroughly, providing a comprehensive answer and clarifying some common misconceptions.
Understanding Physical and Chemical Changes
Before diving into the specifics of boiling, let's establish clear definitions of physical and chemical changes. This foundational knowledge is crucial to accurately classifying the boiling process.
Physical Changes
A physical change alters the form or appearance of a substance but does not change its chemical composition. The molecules themselves remain the same; only their arrangement or state of matter changes. Examples include:
- Changes in state: Melting, freezing, boiling, condensation, sublimation (solid to gas), and deposition (gas to solid).
- Changes in shape: Cutting, bending, crushing.
- Changes in size: Dissolving (in some cases, see below).
- Separation of mixtures: Filtration, distillation.
Crucially, a physical change is reversible. The original substance can be recovered through physical means. For instance, you can freeze water to form ice and then melt the ice back into water.
Chemical Changes
A chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules to form new substances with different properties. The chemical composition of the substance fundamentally alters. Examples include:
- Burning: Combustion reactions involve oxygen and produce new compounds, often gases.
- Rusting: Iron reacts with oxygen and water to form iron oxide (rust).
- Digestion: Complex molecules in food are broken down into simpler ones.
- Cooking an egg: The protein structure changes irreversibly.
A chemical change is generally irreversible. The original substance cannot be easily recovered through simple physical means.
Boiling: A Detailed Analysis
Now let's apply these definitions to the process of boiling. Boiling is the rapid vaporization of a liquid, which occurs when the liquid reaches its boiling point. At this point, the vapor pressure of the liquid equals the atmospheric pressure.
The Molecular Perspective
What happens at the molecular level during boiling? The molecules in a liquid are constantly moving and colliding. As the liquid is heated, the kinetic energy of the molecules increases. This increased energy allows some molecules to overcome the intermolecular forces holding them together in the liquid phase. These molecules escape the liquid's surface and transition into the gaseous phase (steam).
Crucially, the water molecules themselves remain H₂O molecules. There is no change in their chemical composition. Only their state of matter changes from liquid to gas. This is a key indicator of a physical change.
Evidence Supporting Boiling as a Physical Change
Several observations further support the classification of boiling as a physical change:
- Reversible process: If you condense the steam (water vapor) back into liquid water, you obtain the original substance. This reversibility is a hallmark of physical changes.
- No new substances formed: Boiling water simply produces water vapor (steam), which is still chemically H₂O. No new chemical compounds are formed during the process.
- Properties of water remain unchanged: The chemical properties of the water, such as its ability to dissolve certain substances or its reactivity, remain the same before and after boiling.
Addressing Common Misconceptions
Despite the clear evidence, some misconceptions persist about boiling:
- "Boiling changes the taste of water": While prolonged boiling can remove dissolved gases, affecting taste subtly, it doesn't change the chemical composition of the water itself.
- "Boiling purifies water": Boiling does kill many microorganisms, which is a beneficial effect, but this is more a matter of removing contaminants than altering the chemical makeup of the water. The water molecules themselves remain unaltered.
- "Minerals are lost during boiling": Some minerals may precipitate out of solution as the water evaporates, but this is a separation process, not a chemical change to the water molecules. The fundamental chemical composition of the water doesn't change.
Boiling with Dissolved Substances: A More Complex Scenario
The picture becomes slightly more complex when considering boiling solutions – water with dissolved substances. For example, when you boil saltwater, the water evaporates, leaving behind the salt. This might appear to be a chemical change, but it's not.
The salt remains chemically unchanged; it simply separates from the water. The process of evaporation is still a physical change, while the separation of the salt is a physical separation technique. No new chemical compounds are formed during this process.
Conclusion: Boiling is a Physical Change
In conclusion, boiling is unequivocally a physical change. The process involves a change of state, from liquid to gas, but the chemical composition of the substance remains unchanged. While dissolved substances might separate during boiling, this is a physical separation, not a chemical reaction. The water molecules themselves undergo no chemical alteration. The reversibility of the process, the absence of new substance formation, and the preservation of chemical properties all solidify the classification of boiling as a physical change. Understanding this distinction is vital for a firm grasp of fundamental chemistry principles. Remember, while seemingly simple, the boiling process reveals deeper insights into the behavior of matter at a molecular level.
Latest Posts
Latest Posts
-
What Is The Unique Property Of Water
Mar 28, 2025
-
What Is A Particle With A Negative Charge
Mar 28, 2025
-
Why Does Water Have High Heat Of Vaporization
Mar 28, 2025
-
The Type Of Microscope Used In Our Lab Is The
Mar 28, 2025
-
The Neural Tunic Of The Eye
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Is Boil A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
