What Are Rows Called On The Periodic Table
Muz Play
Mar 25, 2025 · 6 min read
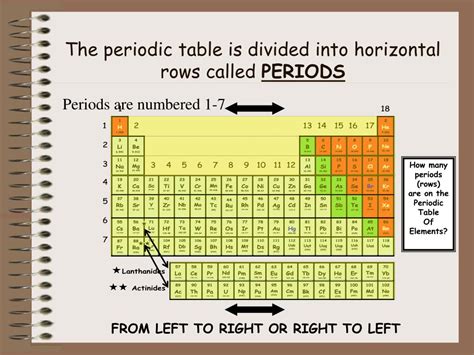
Table of Contents
What Are Rows Called on the Periodic Table? Understanding Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While many are familiar with the table's columns, often called groups or families, the rows also hold significant meaning and are known as periods. Understanding periods is crucial to grasping the trends in element properties and predicting their behavior. This article delves deep into the concept of periods on the periodic table, exploring their significance, the trends they exhibit, and their crucial role in the organization of chemical elements.
Understanding the Structure of the Periodic Table
Before diving into the specifics of periods, let's briefly recap the overall structure of the periodic table. Elements are arranged in order of increasing atomic number, which represents the number of protons in an atom's nucleus. This arrangement is not arbitrary; it reflects the underlying electron configuration of the atoms. The table is organized into rows (periods) and columns (groups or families).
The Significance of Periods
Each period represents a principal energy level, or shell, in an atom. As we move across a period, electrons are progressively added to the same outermost energy level. This addition of electrons influences the chemical properties of the elements within that period. The number of the period corresponds to the highest principal quantum number (n) of the electrons in that element's ground state. For example, elements in Period 1 have electrons only in the n=1 energy level, while elements in Period 2 have electrons in both the n=1 and n=2 energy levels.
The Seven Periods: A Detailed Look
The standard periodic table consists of seven periods, each with a unique number of elements. The number of elements in each period reflects the maximum number of electrons that can occupy the electron shells at a specific energy level.
Period 1: The Shortest Period
Period 1 is exceptionally short, containing only two elements: hydrogen (H) and helium (He). These elements have electrons only in the first energy level (n=1), which can hold a maximum of two electrons. Because of its limited number of elements, Period 1 doesn't exhibit the same pronounced trends as the longer periods.
Period 2 and Period 3: The Second and Third Short Periods
Periods 2 and 3 are also relatively short, containing eight elements each. These elements fill the second (n=2) and third (n=3) energy levels, respectively. Both levels have four orbitals (s and three p orbitals), accommodating a maximum of eight electrons. Within these periods, we begin to see distinct trends in electronegativity, ionization energy, and atomic radius.
Electronegativity: This is the ability of an atom to attract electrons in a chemical bond. Electronegativity generally increases across a period.
Ionization Energy: This is the energy required to remove an electron from an atom. Ionization energy generally increases across a period.
Atomic Radius: This is the distance from the nucleus to the outermost electron. Atomic radius generally decreases across a period.
These trends are explained by the increasing nuclear charge and the limited shielding effect of electrons in the same energy level. As more protons are added across a period, the nucleus' pull on electrons strengthens, leading to smaller atomic radii and higher ionization energies.
Period 4 and Period 5: The Longer Periods, Introducing d-block Elements
Periods 4 and 5 are longer than the previous periods because they include the transition metals (d-block elements). The transition metals fill the 3d and 4d orbitals, respectively, adding ten more elements to these periods. The presence of the d-block elements introduces subtle changes in the trends observed in the earlier periods. The shielding effect of d electrons becomes more significant, affecting the atomic radii and ionization energies. The variation in oxidation states is a characteristic feature of transition metals.
Period 6 and Period 7: The Longest Periods, f-block and the Actinides
Periods 6 and 7 are the longest periods, incorporating the f-block elements (lanthanides and actinides). The lanthanides fill the 4f orbitals, and the actinides fill the 5f orbitals. The f-block elements add a considerable number of elements to these periods, further complicating the trends across the period. The presence of f-electrons significantly influences the chemical and physical properties of these elements, particularly due to their complex electron configurations and shielding effects. The actinides are also known for their radioactivity.
Trends Across a Period: A Detailed Examination
As previously mentioned, several important properties exhibit trends across a period. Understanding these trends is essential for predicting chemical behavior.
Atomic Radius Trend
Atomic radius generally decreases across a period. This is due to the increasing nuclear charge as we move across the period. With more protons, the nucleus exerts a stronger pull on the electrons, leading to a decrease in the atomic radius. However, there are exceptions to this trend, especially within the transition metals, due to the complex electron configurations and shielding effects.
Ionization Energy Trend
Ionization energy generally increases across a period. This is a consequence of the increased nuclear charge and the decrease in atomic radius. It becomes more challenging to remove an electron from an atom with a smaller radius and a stronger nuclear pull. Again, exceptions are observed, particularly in the transition metals due to the effects of electron shielding and electron configurations.
Electronegativity Trend
Electronegativity generally increases across a period. This relates directly to the increasing nuclear charge and decreasing atomic radius. Atoms with higher electronegativity attract electrons more strongly in a chemical bond. Similar to ionization energy and atomic radius, exceptions are observed due to the complexities introduced by the d and f block elements.
Metallic Character Trend
Metallic character generally decreases across a period. Metals tend to lose electrons readily, forming positive ions. As we move across a period, the increased nuclear charge makes it progressively harder for atoms to lose electrons, resulting in a decrease in metallic character. This is evident by the shift from metals to nonmetals across a period.
The Importance of Understanding Periods
Understanding the concept of periods is fundamental to comprehending the periodic table's organization and predicting the behavior of chemical elements. The periodic trends directly reflect the underlying atomic structure and electron configurations of the elements. This knowledge forms the basis for:
- Predicting chemical properties: By understanding the trends across a period, we can predict how elements will react with each other.
- Designing new materials: Knowledge of the periodic trends helps in designing materials with specific properties, such as conductivity, reactivity, or strength.
- Understanding chemical reactions: The periodic table and the concept of periods provide a framework for understanding the mechanisms and outcomes of chemical reactions.
- Developing new technologies: Understanding periods plays a role in technological advancements that rely on manipulating elements' properties.
In summary, the rows of the periodic table – the periods – are far more than a simple arrangement; they provide a profound insight into the structure of matter and the behavior of elements. By understanding the properties and trends exhibited within each period, we unlock a powerful tool for predicting chemical behavior and driving advancements in various scientific fields. From the short periods with their clearly defined trends to the longer periods with the complexities of transition and inner transition metals, the structure of periods is vital for chemical understanding and innovation.
Latest Posts
Latest Posts
-
Calculate The Theoretical Percentage Of Water For The Following Hydrates
Mar 28, 2025
-
Loop Of Wire In A Magnetic Field
Mar 28, 2025
-
If Temperature Increases What Happens To Volume
Mar 28, 2025
-
How To Draw A Titration Curve
Mar 28, 2025
-
How To Find A Euler Circuit
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Are Rows Called On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
