If Temperature Increases What Happens To Volume
Muz Play
Mar 28, 2025 · 6 min read
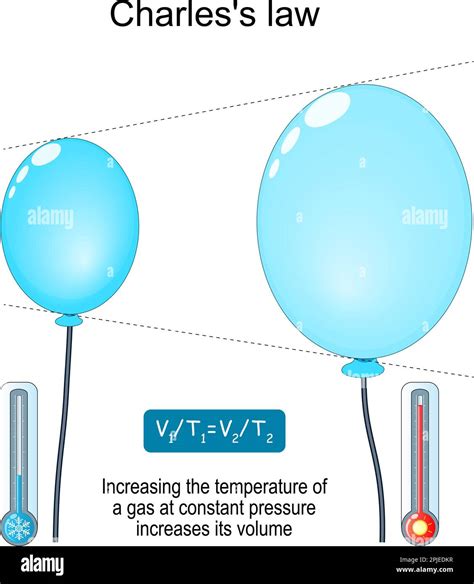
Table of Contents
If Temperature Increases, What Happens to Volume? Exploring Thermal Expansion
The relationship between temperature and volume is fundamental to our understanding of the physical world. As temperature increases, the volume of most substances also increases. This phenomenon, known as thermal expansion, is a direct consequence of the increased kinetic energy of the constituent particles (atoms, molecules, or ions) within the substance. Understanding this relationship is crucial across numerous scientific disciplines, from engineering and materials science to meteorology and cosmology. This article delves deep into the intricacies of thermal expansion, exploring its causes, its variations across different states of matter, and its significant real-world applications and implications.
Understanding Thermal Expansion: The Kinetic Theory Perspective
At the heart of thermal expansion lies the kinetic theory of matter. This theory posits that all matter is composed of tiny particles in constant motion. The kinetic energy of these particles directly relates to the temperature of the substance. When you heat a substance, you increase the average kinetic energy of its particles. This increased kinetic energy translates into more vigorous motion, causing the particles to vibrate, rotate, and translate with greater amplitude.
The Microscopic Dance: Increased Particle Spacing
This increased particle motion leads to a crucial consequence: the average distance between particles increases. Imagine a solid material as a collection of interconnected particles held together by attractive forces. As temperature increases, the particles vibrate more intensely, pushing against these attractive forces. This results in a slight increase in the average separation between particles, leading to an overall expansion in the volume of the material.
The Macroscopic Manifestation: Increased Volume
The microscopic increase in particle spacing translates into a macroscopic change: an increase in the overall volume of the substance. The magnitude of this expansion depends on several factors, including:
- The type of material: Different materials have different coefficients of thermal expansion, reflecting the strength of the interparticle forces and the structure of the material. For instance, metals generally expand more than ceramics, while polymers exhibit even higher expansion rates.
- The initial temperature: The rate of expansion is not always constant; it can vary with temperature.
- The amount of temperature change: A larger temperature increase will result in a greater volume expansion.
Thermal Expansion in Different States of Matter
The relationship between temperature and volume manifests differently across various states of matter:
Solids: A Subtle Shift
In solids, the particles are closely packed and held together by strong intermolecular forces. Therefore, thermal expansion in solids is relatively small. However, it is still significant enough to be considered in many engineering applications, especially for precise measurements and structures. Consider the expansion of bridges and railway lines during summer heat – careful engineering considerations are made to account for this.
Liquids: More Pronounced Expansion
Liquids exhibit more pronounced thermal expansion than solids because their particles are less tightly bound and have greater freedom of movement. The increase in kinetic energy with rising temperature leads to more significant changes in interparticle spacing, resulting in a more noticeable volume increase. This principle is utilized in liquid-in-glass thermometers.
Gases: Dramatic Volume Changes
Gases demonstrate the most significant thermal expansion. The particles in a gas are far apart and experience minimal intermolecular forces. Therefore, an increase in temperature leads to a dramatic increase in the average kinetic energy of the particles, causing them to move much faster and farther apart. This results in a substantial increase in the volume of the gas, especially under constant pressure conditions (as described by Charles's Law).
The Importance of the Coefficient of Thermal Expansion (CTE)
The coefficient of thermal expansion (CTE) is a crucial material property that quantifies the extent to which a material expands or contracts with a change in temperature. It's defined as the fractional change in size per degree Celsius (or Fahrenheit) change in temperature. Materials with high CTEs expand more significantly with temperature increases than materials with low CTEs. The CTE varies with temperature and can be linear, quadratic, or even more complex depending on the material.
Knowing the CTE of a material is essential in engineering and design, as it allows engineers to predict and account for the dimensional changes caused by temperature fluctuations. This is particularly critical in applications involving precision instruments, structures subjected to significant temperature variations (e.g., bridges, pipelines), and composite materials where different components have different CTEs.
Real-World Applications and Implications of Thermal Expansion
The phenomenon of thermal expansion has significant real-world applications and implications:
Engineering and Construction:
- Bridge Construction: Bridges are designed to account for thermal expansion and contraction to prevent structural damage. Expansion joints are incorporated to accommodate the changes in length caused by temperature fluctuations.
- Railway Tracks: Similar to bridges, railway tracks require expansion gaps to prevent buckling due to temperature changes.
- Pipelines: Pipelines carrying liquids or gases need to be designed to withstand thermal expansion and contraction.
- Precision Manufacturing: Thermal expansion must be considered during manufacturing processes that require precise dimensions.
Thermometry and Measurement:
- Liquid-in-Glass Thermometers: The expansion of liquids like mercury or alcohol forms the basis of traditional liquid-in-glass thermometers.
- Bimetallic Strips: Bimetallic strips, composed of two metals with different CTEs, bend when heated due to differential expansion, and this principle is used in thermostats and other temperature-sensitive devices.
Materials Science:
- Material Selection: Understanding CTEs is crucial for selecting appropriate materials for specific applications. For example, materials with low CTEs are preferred in precision instruments where dimensional stability is paramount.
- Composite Materials: The design of composite materials often involves careful selection of components with compatible CTEs to prevent internal stresses and failures due to differential expansion.
Environmental Impacts:
- Climate Change: Thermal expansion of water plays a significant role in sea-level rise. As ocean temperatures increase, the volume of water expands, contributing to the overall rise in sea levels.
- Geological Processes: Thermal expansion and contraction of rocks contribute to weathering and erosion processes, shaping the Earth's surface.
Beyond the Basics: Anomalies and Exceptions
While the general rule is that temperature increases lead to volume increases, there are exceptions and anomalies:
Water's Unusual Behavior:
Water exhibits an unusual behavior between 0°C and 4°C. In this temperature range, water actually contracts as its temperature increases, reaching its maximum density at 4°C. Beyond 4°C, water behaves normally, expanding as the temperature increases. This anomalous behavior is critical for aquatic life, as it prevents bodies of water from freezing solid from the bottom up.
Negative Thermal Expansion:
Some materials exhibit negative thermal expansion, meaning their volume decreases as temperature increases. This phenomenon is relatively rare and is often observed in certain crystalline structures at specific temperature ranges.
Conclusion: A Fundamental Relationship with Far-Reaching Consequences
The relationship between temperature and volume, primarily characterized by thermal expansion, is a fundamental concept with far-reaching consequences across various scientific and engineering disciplines. Understanding the principles of thermal expansion is essential for designing durable structures, developing precise instruments, predicting environmental changes, and advancing our understanding of the behavior of matter at different scales. From the microscopic dance of particles to the macroscopic expansion of bridges and the rise of sea levels, the impact of thermal expansion is pervasive and profound. The careful consideration of this phenomenon is crucial for technological advancements and environmental sustainability alike.
Latest Posts
Latest Posts
-
Is Breaking A Bond Endothermic Or Exothermic
Mar 31, 2025
-
What Are The Three General Characteristics Of Connective Tissue
Mar 31, 2025
-
Blocks Myosin Binding Sites On Actin
Mar 31, 2025
-
Label The Microscopic Structure Of A Skeletal Muscle
Mar 31, 2025
-
What Is The Difference Between Intermolecular Forces And Intramolecular Forces
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about If Temperature Increases What Happens To Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
