Blocks Myosin Binding Sites On Actin
Muz Play
Mar 31, 2025 · 5 min read
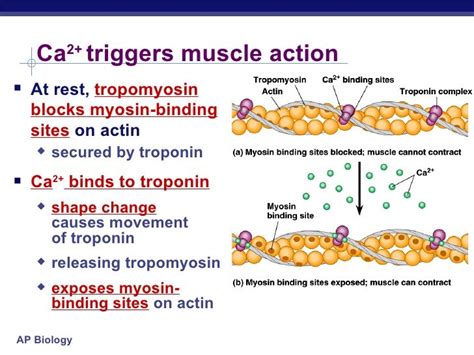
Table of Contents
Blocks Myosin Binding Sites on Actin: A Deep Dive into Muscle Contraction Inhibition
Understanding how muscles contract and relax is fundamental to comprehending human movement and physiology. At the heart of this process lies the intricate interaction between actin and myosin, two key proteins within muscle fibers. This article delves into the fascinating mechanisms that block myosin binding sites on actin, ultimately leading to muscle relaxation and the prevention of unwanted contractions. We'll explore various factors involved, including tropomyosin, troponin, and the role of calcium ions. We will also touch upon the implications of dysregulation in these processes and related diseases.
The Actin-Myosin Interaction: The Basis of Muscle Contraction
Muscle contraction is a complex process driven by the interaction between actin filaments and myosin motor proteins. Actin filaments, thin filaments composed primarily of actin monomers, are arranged in a parallel fashion within the sarcomere, the basic contractile unit of muscle. Myosin filaments, thick filaments with numerous myosin heads, interdigitate with the actin filaments.
The cyclical interaction between actin and myosin involves several key steps:
- Attachment: Myosin heads bind to specific sites on the actin filament, forming cross-bridges.
- Power Stroke: The myosin head undergoes a conformational change, pulling the actin filament towards the center of the sarcomere. This generates force.
- Detachment: ATP binds to the myosin head, causing it to detach from the actin filament.
- Reactivation: ATP is hydrolyzed, resetting the myosin head for another cycle of attachment and power stroke.
This continuous cycle of attachment, power stroke, detachment, and reactivation leads to the sliding of actin and myosin filaments past each other, resulting in muscle shortening and contraction.
The Regulatory Role of Tropomyosin and Troponin: Blocking Myosin Binding Sites
Crucially, the myosin binding sites on actin are not always accessible. In a relaxed muscle, these sites are physically blocked by tropomyosin, a filamentous protein that winds around the actin filament. This blockage prevents myosin from binding and initiating the contraction cycle. Troponin, a complex of three proteins (troponin I, troponin T, and troponin C), plays a crucial role in regulating the position of tropomyosin.
Tropomyosin: The Physical Blockade
Tropomyosin molecules are strategically positioned along the actin filament, covering the myosin-binding sites. This effectively prevents myosin heads from forming cross-bridges and initiating contraction. Imagine it like a switch that's turned off, preventing the muscle from contracting.
Troponin: The Calcium Sensor
Troponin acts as a crucial calcium sensor. Troponin C, a subunit of troponin, has a high affinity for calcium ions (Ca²⁺). When the intracellular calcium concentration increases, Ca²⁺ ions bind to troponin C. This binding triggers a conformational change in the troponin complex, which in turn shifts the position of tropomyosin.
Unmasking the Myosin Binding Sites: The Role of Calcium
The conformational change in troponin caused by Ca²⁺ binding moves tropomyosin away from the myosin-binding sites on actin. This "unmasking" of the binding sites allows myosin heads to attach and initiate the contraction cycle. The increase in intracellular calcium is the trigger that initiates muscle contraction. This intricate interplay between calcium, troponin, and tropomyosin is vital for precisely controlling muscle contraction.
Mechanisms that Block Myosin Binding Sites: Beyond Calcium Regulation
While calcium-mediated regulation through troponin and tropomyosin is the primary mechanism, other factors can influence myosin binding site accessibility and thus muscle contraction:
Titin and Nebulin: Structural Proteins
Titin and nebulin are structural proteins that play important roles in maintaining the organization and integrity of the sarcomere. While not directly blocking myosin binding sites in the same way as tropomyosin, they contribute to the overall structural arrangement which indirectly influences the efficiency of actin-myosin interaction. Dysfunction in these proteins can lead to impaired muscle function.
Other Regulatory Proteins
Several other proteins are involved in regulating muscle contraction, although their effects on myosin binding site accessibility are less direct. These include proteins that modulate the activity of myosin ATPase, affecting the rate of cross-bridge cycling. Research continues to unravel the intricate network of protein interactions controlling muscle function.
Consequences of Impaired Myosin Binding Site Regulation: Muscular Dystrophies and Other Diseases
Disruptions in the mechanisms that regulate myosin binding sites can lead to various muscle-related disorders. Muscular dystrophies, a group of inherited diseases characterized by progressive muscle weakness and degeneration, often involve defects in proteins associated with the actin-myosin interaction. These defects can lead to impaired muscle function and eventually muscle fiber death.
Examples of Related Diseases:
- Duchenne Muscular Dystrophy (DMD): This severe form of muscular dystrophy is caused by mutations in the dystrophin gene, leading to instability of the muscle fiber membrane and impaired muscle function. While not directly blocking myosin binding sites, the resulting muscle damage indirectly impacts the interaction between actin and myosin.
- Becker Muscular Dystrophy (BMD): A milder form of muscular dystrophy, BMD is also caused by mutations in the dystrophin gene, but the mutations are less severe than in DMD.
- Myotonic Dystrophy: This is characterized by muscle weakness and myotonia (prolonged muscle contractions). It's caused by defects in genes responsible for RNA metabolism, indirectly affecting muscle protein function.
Therapeutic Interventions: Targeting Muscle Contraction
Understanding the mechanisms that regulate myosin binding sites is critical for developing therapeutic interventions for muscle-related diseases. Research is focused on several strategies, including:
- Gene therapy: Replacing defective genes responsible for muscular dystrophies.
- Pharmacological interventions: Developing drugs that modulate the activity of proteins involved in muscle contraction.
- Stem cell therapy: Replacing damaged muscle fibers with healthy ones.
Conclusion: A Complex and Dynamic Process
The regulation of myosin binding sites on actin is a highly complex and dynamic process crucial for muscle function. The intricate interplay between tropomyosin, troponin, calcium ions, and other regulatory proteins ensures precise control over muscle contraction and relaxation. Disruptions in these mechanisms can lead to various muscle-related diseases, highlighting the importance of further research to develop effective therapeutic interventions. Continued investigation into the intricacies of this interaction promises to yield new insights into muscle physiology and pave the way for more effective treatments for muscle disorders. The understanding of these intricate mechanisms is not just fundamental to biology but also critical for the development of advanced therapies for a variety of muscle diseases. The future holds promise for more targeted therapies that will improve the quality of life for individuals affected by muscle disorders.
Latest Posts
Latest Posts
-
Examples Of Instantaneous Rate Of Change
Apr 01, 2025
-
How Is The Use Of Symbols Related To Culture
Apr 01, 2025
-
As You Move Across The Periodic Table
Apr 01, 2025
-
What Is In The Atmosphere Of Jupiter
Apr 01, 2025
-
What Does A Negative Reduction Potential Mean
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Blocks Myosin Binding Sites On Actin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
