As You Move Across The Periodic Table
Muz Play
Apr 01, 2025 · 6 min read
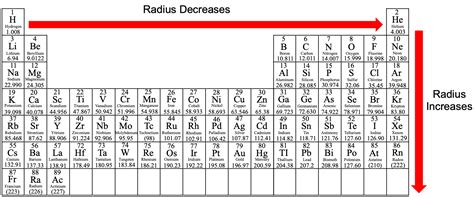
Table of Contents
As You Move Across the Periodic Table: Trends in Atomic Properties
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding the trends that emerge as you move across a period (row) or down a group (column) is crucial for predicting and explaining chemical behavior. This article will delve into the fascinating patterns observed as you traverse the periodic table horizontally, focusing on atomic radius, ionization energy, electron affinity, and electronegativity.
Atomic Radius: Getting Smaller Across a Period
Atomic radius refers to the size of an atom, typically measured as half the distance between the nuclei of two identical atoms bonded together. As you move from left to right across a period, the atomic radius generally decreases. This trend arises from several factors:
The Role of Effective Nuclear Charge
The effective nuclear charge (Z<sub>eff</sub>) is the net positive charge experienced by an electron in an atom. It's the difference between the number of protons in the nucleus and the shielding effect of inner electrons. As you move across a period, the number of protons increases, leading to a stronger positive charge in the nucleus. Simultaneously, the added electrons are entering the same principal energy level (shell), and their shielding effect is relatively limited. This results in a higher effective nuclear charge, pulling the outer electrons closer to the nucleus and shrinking the atomic radius.
Shielding Effect: Inner Electrons' Influence
The shielding effect, caused by inner electrons, reduces the attractive force between the nucleus and outer electrons. While the number of protons increases, the shielding effect from the inner electrons remains relatively constant within a period, thus allowing Z<sub>eff</sub> to dominate the size of the atom.
Exceptions and Irregularities
While the general trend is a decrease in atomic radius across a period, minor irregularities can occur. These exceptions are often due to electron-electron repulsions within the same subshell, slightly counteracting the pull of the increasing nuclear charge.
Ionization Energy: The Energy to Remove an Electron
Ionization energy (IE) is the minimum energy required to remove an electron from a neutral gaseous atom. The first ionization energy (IE<sub>1</sub>) refers to the removal of the first electron, the second ionization energy (IE<sub>2</sub>) refers to the removal of the second electron, and so on. As you move across a period, ionization energy generally increases.
The Effect of Increasing Effective Nuclear Charge
The increase in ionization energy across a period directly correlates with the increase in effective nuclear charge. The stronger the attraction between the nucleus and the electrons, the more energy is required to remove an electron.
Electron Configuration and Stability
The electronic configuration also plays a role. Elements with filled or half-filled subshells exhibit relatively high ionization energies due to their enhanced stability. For example, the ionization energy of nitrogen is higher than oxygen despite oxygen having a higher effective nuclear charge. This is because nitrogen has a half-filled p subshell, adding stability.
Successive Ionization Energies
It's important to note that successive ionization energies (IE<sub>2</sub>, IE<sub>3</sub>, etc.) increase significantly more than the first ionization energy. This is because removing an electron from a positively charged ion requires overcoming a stronger electrostatic attraction.
Electron Affinity: Attraction for an Electron
Electron affinity (EA) is the energy change associated with adding an electron to a neutral gaseous atom. While not as straightforward as ionization energy, a general trend exists across a period: Electron affinity generally increases (becomes more negative) as you move across a period. This means that atoms generally become more willing to accept an extra electron.
The Influence of Effective Nuclear Charge
The increased effective nuclear charge across a period leads to a stronger attraction for an additional electron. This explains the general trend of increasing electron affinity.
Exceptions and Stability
Similar to ionization energy, exceptions to this general trend exist, often related to the stability of the resulting electron configuration. For instance, the electron affinity of the noble gases is exceptionally low (positive), as adding an electron would disrupt their stable, filled electron shells.
Electronegativity: Sharing or Stealing Electrons
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Like ionization energy and electron affinity, electronegativity generally increases as you move across a period.
Relationship to Effective Nuclear Charge
The higher effective nuclear charge across a period leads to a greater pull on shared electrons in a covalent bond, resulting in higher electronegativity.
Predicting Bond Polarity
Electronegativity is crucial for predicting the polarity of chemical bonds. The larger the difference in electronegativity between two atoms, the more polar the bond will be.
Summary of Periodic Trends Across a Period
| Property | Trend Across a Period | Explanation |
|---|---|---|
| Atomic Radius | Decreases | Increasing effective nuclear charge |
| Ionization Energy | Increases | Increasing effective nuclear charge, stability of electron configurations |
| Electron Affinity | Generally Increases (more negative) | Increasing effective nuclear charge, exceptions due to electronic configuration |
| Electronegativity | Increases | Increasing effective nuclear charge |
Beyond the Basic Trends: A Deeper Dive
The trends discussed above provide a foundational understanding of the behavior of elements across the periodic table. However, a complete picture requires considering additional factors:
Transition Metals: Irregularities in Trends
The transition metals, located in the d-block of the periodic table, exhibit more complex trends than the main group elements (s- and p-blocks). While the general trends of decreasing atomic radius and increasing ionization energy still apply, the differences are less pronounced due to the complex shielding effects of d-electrons.
Lanthanides and Actinides: f-block Complications
The lanthanides and actinides, located in the f-block, show even more subtle variations in their properties. The poor shielding by f-electrons leads to complex interactions and less predictable trends.
The Importance of Electron Configuration
The electronic configuration of an element plays a critical role in determining its properties. Elements with fully or half-filled subshells often deviate from the general trends due to their increased stability.
Applications of Understanding Periodic Trends
Understanding these trends is essential in various fields:
Predicting Chemical Reactions
Knowing the relative electronegativities of atoms helps predict the polarity of bonds and the reactivity of molecules.
Designing New Materials
The properties of elements, influenced by their position on the periodic table, are crucial in designing materials with specific characteristics for diverse applications.
Understanding Biological Systems
The elements essential for life, and their interactions, are heavily influenced by their position on the periodic table.
Developing New Technologies
Many technological advancements rely on understanding and manipulating the properties of elements, guided by periodic trends.
Conclusion: A Journey Across the Table
The periodic table is more than just a list of elements; it's a powerful tool for predicting and explaining the behavior of matter. By understanding the trends in atomic properties as you move across a period, we can gain valuable insights into the chemical world, from the reactivity of individual atoms to the design of complex materials and technologies. The journey across the periodic table is a journey into the heart of chemistry, revealing the fundamental principles that govern the world around us. Further exploration into the specific properties of individual elements and groups will only deepen this understanding. Remember, the periodic table is a dynamic and ever-evolving landscape, continuously revealing new facets of the chemical universe.
Latest Posts
Latest Posts
-
Monomers Are Connected In What Type Of Reaction
Apr 02, 2025
-
Find The Equation Of The Vertical Line
Apr 02, 2025
-
Electron Configuration For Copper And Chromium
Apr 02, 2025
-
Confidence Interval Calculator With Two Samples
Apr 02, 2025
-
Lewis Base Vs Bronsted Lowry Base
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about As You Move Across The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
