Is Breaking A Bond Endothermic Or Exothermic
Muz Play
Mar 31, 2025 · 5 min read
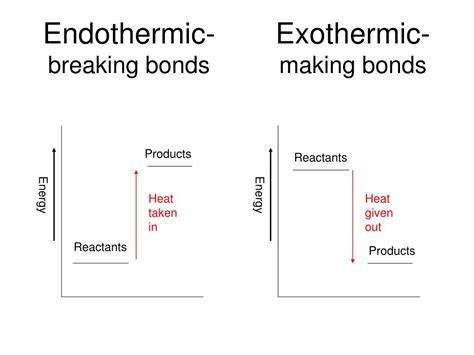
Table of Contents
Is Breaking a Bond Endothermic or Exothermic? Understanding Bond Energies and Enthalpy Changes
The question of whether breaking a chemical bond is endothermic or exothermic is fundamental to understanding chemical reactions and thermodynamics. The short answer is: breaking a chemical bond is always endothermic. This means it requires energy input to break the bond. Conversely, forming a chemical bond is always exothermic, releasing energy. Let's delve deeper into the concepts and explore why this is the case.
Understanding Endothermic and Exothermic Processes
Before we tackle the specifics of bond breaking, it's crucial to understand the difference between endothermic and exothermic processes.
-
Endothermic processes: These processes absorb energy from their surroundings. The system's energy increases, and the surroundings become cooler. Think of melting ice – you need to add heat (energy) to break the bonds holding the water molecules in a solid structure.
-
Exothermic processes: These processes release energy to their surroundings. The system's energy decreases, and the surroundings become warmer. Think of burning wood – the combustion reaction releases heat (energy) as new bonds form.
Bond Energy: The Key to Understanding Bond Breaking
The strength of a chemical bond is quantified by its bond energy (or bond dissociation energy). This is the amount of energy required to break one mole of a particular type of bond in the gaseous phase. Bond energy is always a positive value because energy must be added to break the bond.
Units of Bond Energy
Bond energy is typically expressed in kilojoules per mole (kJ/mol). This means it represents the energy required to break one mole (6.022 x 10<sup>23</sup>) of bonds.
Factors Affecting Bond Energy
Several factors influence the bond energy:
-
Bond Order: Higher bond order (e.g., triple bond > double bond > single bond) signifies stronger bonds requiring more energy to break. A triple bond shares three electron pairs, making it considerably stronger than a single bond.
-
Bond Length: Shorter bonds are generally stronger bonds. The closer the atoms are, the stronger the electrostatic attraction between them, resulting in higher bond energy.
-
Electronegativity: The difference in electronegativity between the atoms involved in the bond affects the bond strength. Larger electronegativity differences can lead to more polar bonds, which may be slightly stronger or weaker depending on other factors. This is more complex than simple bond order and length.
-
Hybridization: The type of hybrid orbitals involved in bond formation also influences bond energy. For example, sp hybridized orbitals form stronger bonds than sp<sup>3</sup> hybridized orbitals.
Why Breaking a Bond is Endothermic: A Deeper Look
To truly understand why breaking a bond is endothermic, we need to consider the forces holding the atoms together. These are primarily electrostatic forces of attraction between the positively charged nuclei and the negatively charged electrons shared in the bond.
Breaking a bond involves overcoming these attractive forces. Energy must be supplied to separate the atoms against these attractive forces, hence the endothermic nature of the process. Think of it like pulling two magnets apart – you need to exert force (energy) to overcome their attraction.
The Relationship Between Bond Breaking, Bond Formation, and Enthalpy Change (ΔH)
The enthalpy change (ΔH) of a reaction reflects the overall energy change during the process. For a reaction involving bond breaking and bond formation, the overall ΔH is the sum of the energy required to break bonds (endothermic) and the energy released when new bonds form (exothermic).
ΔH<sub>reaction</sub> = Σ(bond energies of bonds broken) - Σ(bond energies of bonds formed)
If the energy required to break bonds is greater than the energy released when new bonds are formed, the overall reaction is endothermic (ΔH > 0). Conversely, if the energy released during bond formation outweighs the energy required to break bonds, the reaction is exothermic (ΔH < 0).
Examples Illustrating Endothermic Bond Breaking
Let's consider a few examples to further illustrate the concept:
1. Dissociation of a Diatomic Molecule:
The dissociation of a diatomic molecule like hydrogen (H<sub>2</sub>) into two hydrogen atoms (2H) is a classic example of an endothermic process. The H-H bond must be broken, requiring energy input.
H<sub>2</sub>(g) → 2H(g) ΔH > 0
2. Decomposition Reactions:
Many decomposition reactions involve breaking bonds and are endothermic. For example, the decomposition of calcium carbonate (CaCO<sub>3</sub>) into calcium oxide (CaO) and carbon dioxide (CO<sub>2</sub>) requires energy input to break the bonds within the CaCO<sub>3</sub> structure.
CaCO<sub>3</sub>(s) → CaO(s) + CO<sub>2</sub>(g) ΔH > 0
3. Vaporization:
Vaporization, the process of a liquid changing into a gas, involves breaking intermolecular forces (like hydrogen bonds or van der Waals forces) between molecules. This process requires energy input and is thus endothermic.
H<sub>2</sub>O(l) → H<sub>2</sub>O(g) ΔH > 0
Applications and Importance
Understanding the endothermic nature of bond breaking has significant implications across various fields:
-
Chemistry: Predicting the enthalpy changes of reactions based on bond energies. Designing reactions to either absorb or release specific amounts of heat.
-
Materials Science: Developing new materials with desired properties by manipulating bond energies and strengths. Understanding how materials break down or degrade under different conditions.
-
Biology: Understanding the energy requirements of metabolic processes, which frequently involve bond breaking and formation. Studying enzyme catalysis, which facilitates bond breaking and formation in biological systems.
-
Environmental Science: Studying atmospheric reactions, understanding the energy changes involved in greenhouse gas formation and breakdown.
Conclusion
Breaking a chemical bond is an inherently endothermic process. This fundamental concept underpins our understanding of chemical reactions and energy changes. By understanding bond energies and the factors influencing them, we can predict whether reactions will be endothermic or exothermic and gain insights into a wide range of chemical and physical processes. The energy required to break bonds is a critical factor in determining the overall enthalpy change of a reaction, influencing everything from the design of chemical processes to our understanding of biological systems and environmental phenomena. Remember that while breaking bonds is always endothermic, the overall reaction can still be exothermic if the energy released from forming new bonds is greater than the energy consumed in breaking the old ones.
Latest Posts
Latest Posts
-
When Does The Segregation Of Alleles Occur
Apr 01, 2025
-
Cardiac Muscles Differ From Skeletal Muscles In That They
Apr 01, 2025
-
Closing Entries Are Journalized And Posted
Apr 01, 2025
-
How To Calculate Parts Per Thousand
Apr 01, 2025
-
Which Of The Following Statements Pertaining To Asthma Is False
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Breaking A Bond Endothermic Or Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
