Why Are Amino Acids Called Amino Acids
Muz Play
Mar 23, 2025 · 6 min read
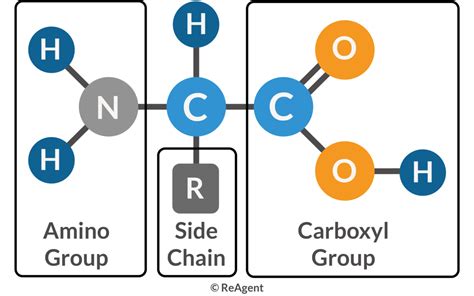
Table of Contents
Why Are Amino Acids Called Amino Acids? A Deep Dive into the Chemistry of Life's Building Blocks
Amino acids. The very name conjures images of complex biological machinery, intricate protein folding, and the fundamental building blocks of life. But have you ever stopped to consider the why behind the name? Why are these crucial molecules called amino acids? Understanding this requires a journey into the fascinating world of organic chemistry, exploring their unique structure and the properties that define them. This article will delve deep into the etymology of "amino acid," explaining the chemical components that earned them this name and exploring their significance in biological systems.
The "Amino" Part: Understanding the Amine Group
The prefix "amino" directly points to the presence of an amine group (-NH₂). This functional group is characterized by a nitrogen atom bonded to two hydrogen atoms. It's this nitrogen-containing group that grants amino acids their basic properties. The amine group's lone pair of electrons on the nitrogen atom allows it to readily accept a proton (H⁺), acting as a weak base. This basicity plays a crucial role in the amino acid's behavior in different pH environments, affecting its charge and interactions with other molecules.
The Importance of the Amine Group in Amino Acid Function:
-
Protein Structure: The amine group participates in the formation of peptide bonds, which link amino acids together to form polypeptide chains – the precursors to proteins. The peptide bond forms between the carboxyl group of one amino acid and the amine group of another, releasing a water molecule in the process. This fundamental reaction is responsible for the vast diversity of protein structures and functions.
-
Enzyme Activity: The amine group can directly participate in enzymatic reactions, often acting as a nucleophile, attacking electrophilic centers on other molecules. This is crucial for various metabolic processes and cellular functions.
-
pH Regulation: The amine group’s ability to accept or donate protons contributes to the amino acid's buffering capacity, helping to maintain a stable pH within cells.
The "Acid" Part: The Carboxylic Acid Group
The suffix "-acid" refers to the presence of a carboxylic acid group (-COOH). This functional group consists of a carbon atom double-bonded to an oxygen atom and single-bonded to a hydroxyl group (-OH). This group is responsible for the acidic properties of amino acids. The hydroxyl group's hydrogen atom can be easily released as a proton (H⁺), making the carboxylic acid a weak acid. This acidity influences the amino acid's behavior in solution and its interactions with other molecules.
The Role of the Carboxylic Acid Group in Amino Acid Function:
-
Peptide Bond Formation: As mentioned earlier, the carboxylic acid group is essential for the formation of peptide bonds, linking amino acids into protein chains. The carboxyl group's ability to lose a proton facilitates this crucial process.
-
Enzyme Active Sites: The carboxylic acid group can also be directly involved in enzyme active sites, providing a binding site for substrates or participating in catalytic mechanisms.
-
Metabolic Pathways: Carboxylic acid groups are frequently involved in various metabolic pathways, participating in reactions such as decarboxylation (removal of the carboxyl group) or esterification (formation of an ester bond).
The α-Carbon: The Central Hub
Both the amine group and the carboxylic acid group are attached to a central carbon atom, known as the α-carbon. This carbon atom is also bonded to a hydrogen atom and a side chain (also called an R group), which is unique to each amino acid. The variation in these side chains accounts for the remarkable diversity of amino acids, each with its own distinct chemical and physical properties.
The Significance of the Side Chain (R Group):
-
Chemical Diversity: The side chain can be anything from a simple hydrogen atom (as in glycine) to complex aromatic rings (as in phenylalanine) or even charged groups (as in aspartic acid or lysine). This diversity directly influences the amino acid's properties, such as polarity, charge, size, and hydrophobicity.
-
Protein Folding: The side chains' interactions (hydrophobic interactions, hydrogen bonding, ionic bonds, disulfide bridges) dictate how the protein folds into its three-dimensional structure. This structure is crucial for its function.
-
Protein Function: The properties of the side chains determine how the protein interacts with other molecules, including substrates, receptors, and other proteins.
Beyond the Basic Structure: Exploring Amino Acid Classification
The presence of the amine and carboxylic acid groups attached to the α-carbon is the defining characteristic of all amino acids. However, amino acids can be further classified based on the properties of their side chains:
1. Nonpolar, Aliphatic Amino Acids:
These amino acids have nonpolar, hydrocarbon side chains. They are generally hydrophobic and tend to cluster together in the interior of proteins, away from the aqueous environment. Examples include glycine, alanine, valine, leucine, isoleucine, and methionine.
2. Aromatic Amino Acids:
These amino acids possess aromatic rings in their side chains. They are relatively nonpolar but can participate in some weak interactions, such as pi-stacking. Examples include phenylalanine, tyrosine, and tryptophan.
3. Polar, Uncharged Amino Acids:
These amino acids have polar, but uncharged, side chains. They can form hydrogen bonds with water and other polar molecules. Examples include serine, threonine, cysteine, asparagine, and glutamine.
4. Positively Charged (Basic) Amino Acids:
These amino acids have side chains with a positive charge at physiological pH. They readily interact with negatively charged molecules. Examples include lysine, arginine, and histidine.
5. Negatively Charged (Acidic) Amino Acids:
These amino acids possess side chains with a negative charge at physiological pH. They strongly interact with positively charged molecules. Examples include aspartic acid and glutamic acid.
The Importance of Amino Acids in Biological Systems
Understanding why amino acids are called amino acids is just the first step. The true significance lies in their pivotal role in virtually all biological processes:
-
Protein Synthesis: Amino acids are the monomers that are linked together to form proteins, the workhorses of the cell. Proteins perform a vast array of functions, including catalysis (enzymes), transport, structure, defense, and signaling.
-
Neurotransmitter Synthesis: Several amino acids, such as glutamate, GABA, glycine, and aspartate, serve as neurotransmitters, transmitting signals between nerve cells.
-
Metabolic Pathways: Amino acids are involved in numerous metabolic pathways, providing building blocks for other molecules or serving as energy sources.
-
Hormone Production: Some amino acids are precursors to hormones, such as thyroxine (thyroid hormone) and melatonin.
Conclusion: The Name Reflects the Function
The name "amino acid" accurately reflects the chemical structure of these fundamental molecules. The presence of both an amine group and a carboxylic acid group, linked to a central α-carbon and a diverse side chain, is what defines them. This unique structure is responsible for their remarkable chemical properties and their crucial roles in building and maintaining life itself. From the simple peptide bond to the complex architecture of proteins, the seemingly straightforward name "amino acid" encapsulates the essence of these extraordinary molecules, the building blocks of life's intricate machinery. The next time you encounter this term, remember the rich chemistry behind it, and appreciate the sophisticated role these molecules play in the incredible complexity of life.
Latest Posts
Latest Posts
-
First Formulation Of The Categorical Imperative
Mar 25, 2025
-
Rearrangement Of Benzil To Benzilic Acid
Mar 25, 2025
-
Is Table Salt A Mixture Or Pure Substance
Mar 25, 2025
-
How To Do Inverse Laplace Transforms
Mar 25, 2025
-
Real World Application Of A Linear Equation In 2 Variables
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Why Are Amino Acids Called Amino Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
