Rearrangement Of Benzil To Benzilic Acid
Muz Play
Mar 25, 2025 · 5 min read
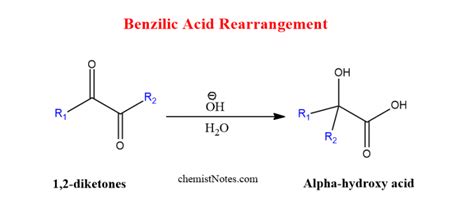
Table of Contents
The Benzilic Acid Rearrangement: A Deep Dive into Mechanism, Applications, and Synthetic Utility
The benzilic acid rearrangement is a fascinating name reaction in organic chemistry, showcasing a captivating transformation of α-diketones into α-hydroxycarboxylic acids. This rearrangement, driven by a base-catalyzed nucleophilic addition followed by a 1,2-shift, offers a unique synthetic route to access valuable building blocks and intricate molecular architectures. This comprehensive article delves into the intricacies of this reaction, exploring its mechanism, variations, applications, and synthetic utility in detail.
Understanding the Benzilic Acid Rearrangement: A Mechanistic Overview
The classic benzilic acid rearrangement involves the conversion of benzil (diphenylglyoxal) to benzilic acid (2-hydroxy-2,2-diphenylacetic acid) under basic conditions. This transformation is not merely a simple substitution but a complex rearrangement involving several key steps.
Step 1: Nucleophilic Attack
The reaction initiates with a nucleophilic attack of a hydroxide ion (OH⁻) on one of the carbonyl carbons of benzil. This attack leads to the formation of a tetrahedral intermediate. This intermediate is crucial, as it sets the stage for the subsequent rearrangement steps.
Step 2: Intramolecular Proton Transfer
Within the tetrahedral intermediate, an intramolecular proton transfer occurs. A proton from the hydroxyl group is transferred to one of the negatively charged oxygen atoms. This step is essential for the rearrangement to proceed. It leads to a more stable intermediate, facilitating the following 1,2-shift.
Step 3: 1,2-Migration
The core of the benzilic acid rearrangement is a 1,2-aryl shift. One of the phenyl groups migrates from the carbonyl carbon to the adjacent carbon bearing the hydroxyl group. This migration step is the rate-determining step and is facilitated by the presence of the electron-withdrawing carbonyl group. This shift forms a new carbon-carbon bond and breaks the existing carbon-carbon bond.
Step 4: Protonation and Tautomerization
The intermediate formed after the 1,2-migration is then protonated by a water molecule. This protonation step completes the rearrangement, yielding the benzilic acid anion. Finally, the acid-base equilibrium results in the formation of the neutral benzilic acid.
Factors Influencing the Benzilic Acid Rearrangement
Several factors significantly influence the efficiency and yield of the benzilic acid rearrangement. Understanding these factors is crucial for optimizing the reaction conditions and obtaining high yields of the desired product.
Choice of Base
The strength of the base used plays a critical role. Strong bases, such as hydroxide ions (OH⁻), potassium hydroxide (KOH), or sodium hydroxide (NaOH), are commonly employed. The concentration of the base also affects the reaction rate. Higher concentrations generally lead to faster reaction rates but can sometimes lead to side reactions.
Solvent Selection
The solvent system employed significantly impacts the reaction kinetics and yield. Protic solvents like water, ethanol, or methanol are often preferred, as they facilitate the proton transfer steps involved in the mechanism. Aprotic solvents can also be used, but the reaction rate might be slower.
Temperature
The temperature also affects the reaction rate. Elevated temperatures generally accelerate the reaction, but excessively high temperatures can lead to side reactions or decomposition of the reactants. Optimizing the temperature is crucial for achieving high yields.
Substrate Structure
The structure of the α-diketone substrate significantly influences the reaction outcome. Symmetrical α-diketones, like benzil, typically undergo the rearrangement smoothly. However, unsymmetrical α-diketones can give rise to a mixture of products, as the migration can occur from either side. The migratory aptitude of the aryl groups also influences the regioselectivity of the reaction. Electron-donating groups on the aryl rings enhance the migratory aptitude, while electron-withdrawing groups diminish it.
Variations and Extensions of the Benzilic Acid Rearrangement
The benzilic acid rearrangement has been extended to a variety of substrates beyond the classic benzil to benzilic acid conversion.
Unsymmetrical α-Diketones
As mentioned earlier, unsymmetrical α-diketones lead to a mixture of products. The ratio of products is determined by the relative migratory aptitudes of the substituents. Predicting the major product requires considering the electronic effects of substituents on the migrating group.
Cyclic α-Diketones
Cyclic α-diketones also undergo the benzilic acid rearrangement, forming cyclic α-hydroxycarboxylic acids. This variation often provides access to valuable chiral building blocks. The ring size can affect the reaction rate and yield.
Aliphatic α-Diketones
While less common, aliphatic α-diketones can also undergo the rearrangement, though often with lower yields compared to aromatic counterparts. This is partly due to the lower stability of the aliphatic intermediates.
Applications and Synthetic Utility
The benzilic acid rearrangement has found several applications in organic synthesis and beyond.
Synthesis of Pharmaceuticals and Natural Products
The rearrangement has been employed in the synthesis of various pharmaceuticals and natural products, providing a crucial step in accessing complex molecular architectures. Its ability to generate chiral centers makes it particularly useful in asymmetric synthesis.
Polymer Chemistry
The benzilic acid rearrangement has also found utility in polymer chemistry. Modified versions of the rearrangement are employed in polymerization reactions, creating new polymer structures with unique properties.
Material Science
The synthetic versatility of the benzilic acid rearrangement also extends into material science. It's used in designing novel materials with specific physical and chemical characteristics, tailoring properties for specific applications.
Conclusion: A Versatile Reaction with Lasting Significance
The benzilic acid rearrangement stands as a testament to the elegance and power of name reactions in organic chemistry. Its mechanistic simplicity, coupled with its versatility and wide range of applications, ensures its continued significance in organic synthesis, providing access to a vast array of valuable molecules. Further investigation into variations, optimization techniques, and exploration of novel applications will undoubtedly solidify its enduring role in the future of synthetic chemistry.
Further Exploration and Research Directions
Future research into the benzilic acid rearrangement could explore several promising avenues:
- Catalysis: Developing new and more efficient catalysts could further improve reaction yields and selectivity. This includes exploring organocatalysis and metal catalysis for the rearrangement.
- Asymmetric Synthesis: Expanding on the use of the rearrangement in asymmetric synthesis, targeting the creation of chiral centers with high enantioselectivity.
- Green Chemistry: Exploring the use of greener solvents and reagents to make the reaction more environmentally friendly.
- Computational Studies: Employing computational techniques to better understand the reaction mechanism and to predict reaction outcomes for various substrates.
- New Substrates: Exploring the rearrangement with novel substrates to expand the scope of the reaction and discover new synthetic pathways.
By continuing to investigate these avenues, we can unlock the full potential of this classic reaction and continue to leverage its power in addressing modern synthetic challenges. The benzilic acid rearrangement, even after years of study, remains a rich area for exploration and discovery, promising further advancements in the field of organic chemistry.
Latest Posts
Latest Posts
-
What Is The Average Kinetic Energy
Mar 25, 2025
-
What Are The Building Blocks Of Macromolecules
Mar 25, 2025
-
Chemistry The Molecular Nature Of Matter And Change 8th Edition
Mar 25, 2025
-
Which Event Always Involves A Chemical Change
Mar 25, 2025
-
What Happens When An Atom Gains An Electron
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Rearrangement Of Benzil To Benzilic Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
