What Is The Average Kinetic Energy
Muz Play
Mar 25, 2025 · 6 min read
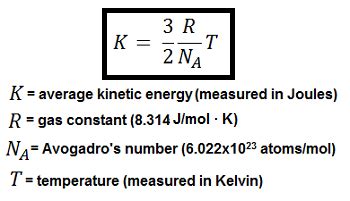
Table of Contents
What is Average Kinetic Energy? A Deep Dive into Molecular Motion and its Implications
Understanding average kinetic energy is crucial for grasping fundamental concepts in physics and chemistry. It's a cornerstone of the kinetic theory of gases, impacting everything from the behavior of ideal gases to the understanding of temperature and its relationship to molecular motion. This comprehensive guide will explore average kinetic energy, its calculation, implications, and applications across various scientific fields.
What is Kinetic Energy?
Before diving into average kinetic energy, let's clarify the concept of kinetic energy itself. Kinetic energy is the energy an object possesses due to its motion. A stationary object has zero kinetic energy. The faster an object moves and the more massive it is, the greater its kinetic energy. The formula for kinetic energy (KE) is:
KE = 1/2 * m * v²
where:
- m represents the mass of the object (in kilograms, kg)
- v represents the velocity of the object (in meters per second, m/s)
The Concept of Average Kinetic Energy
In a system containing many particles, like a gas in a container, each particle possesses its own kinetic energy. These particles move with a range of velocities due to constant collisions and interactions. Therefore, it's impractical to consider the kinetic energy of each individual particle. Instead, we use the concept of average kinetic energy to characterize the system's overall energy state.
Average kinetic energy (AKE) is the average kinetic energy of all the particles in a system. It's a statistical measure reflecting the typical kinetic energy of a particle within that system. This average is particularly useful when dealing with large ensembles of particles, such as in the study of gases, liquids, and solids.
Calculating Average Kinetic Energy
The calculation of average kinetic energy depends on the system's nature. For an ideal gas, the average kinetic energy is directly related to its absolute temperature. This relationship is described by the following equation:
AKE = (3/2) * k * T
Where:
- AKE is the average kinetic energy of a particle
- k is the Boltzmann constant (approximately 1.38 x 10⁻²³ J/K)
- T is the absolute temperature of the gas (in Kelvin, K)
This equation highlights a crucial link between temperature and molecular motion. A higher temperature implies a higher average kinetic energy, meaning the gas particles move faster on average. Conversely, a lower temperature translates to slower particle movement and lower average kinetic energy. This principle is fundamental to understanding thermal phenomena.
Derivation of the Average Kinetic Energy Formula for Ideal Gases
The derivation of the (3/2)kT formula involves statistical mechanics and the Maxwell-Boltzmann distribution, which describes the probability of finding a particle with a specific velocity at a given temperature. It's a more advanced topic, but the key takeaway is that this formula emerges from considering the three-dimensional motion of gas particles and their distribution of velocities. The factor of 3 accounts for the three degrees of freedom (movement in x, y, and z directions) of a monatomic ideal gas.
Implications and Applications of Average Kinetic Energy
The concept of average kinetic energy has far-reaching implications across various scientific domains:
1. Kinetic Theory of Gases:
The kinetic theory of gases utilizes the average kinetic energy to explain macroscopic gas properties like pressure and temperature. The pressure exerted by a gas arises from the countless collisions of gas particles with the container walls. The frequency and force of these collisions are directly related to the average kinetic energy of the gas particles.
2. Understanding Temperature:
Temperature is a direct measure of the average kinetic energy of the particles within a substance. A higher temperature reflects faster particle motion and higher average kinetic energy. This connection underpins our understanding of heat transfer, as heat flows from regions of higher average kinetic energy to regions of lower average kinetic energy.
3. Chemical Reactions:
Average kinetic energy plays a crucial role in chemical reactions. For a reaction to occur, reactant molecules must collide with sufficient energy, often exceeding a minimum energy threshold known as the activation energy. The higher the average kinetic energy (higher temperature), the greater the likelihood of successful collisions and faster reaction rates. This is why heating reactants often accelerates chemical reactions.
4. Diffusion and Effusion:
The rate at which gases diffuse (spread out) or effuse (escape through a small opening) is related to their average kinetic energy. Gases with higher average kinetic energies (lighter gases at a given temperature) diffuse and effuse faster than gases with lower average kinetic energies (heavier gases at a given temperature). This is explained by Graham's Law of Diffusion and Effusion.
5. Phase Transitions:
Changes in the state of matter (solid, liquid, gas) are influenced by average kinetic energy. Adding heat increases the average kinetic energy of particles, eventually overcoming intermolecular forces and leading to phase transitions like melting (solid to liquid) or boiling (liquid to gas).
6. Statistical Mechanics:
Average kinetic energy is a central concept in statistical mechanics, a branch of physics that uses statistical methods to describe the behavior of large ensembles of particles. It allows for the prediction of macroscopic properties from microscopic behavior.
Beyond Ideal Gases: Real Gases and Other Systems
While the (3/2)kT formula applies well to ideal gases, it's an approximation. Real gases deviate from ideal behavior, particularly at high pressures and low temperatures. Intermolecular forces and the finite size of gas molecules become significant under such conditions, altering the average kinetic energy calculation. More complex equations are necessary to account for these deviations.
Furthermore, the concept of average kinetic energy applies beyond gases. It can be extended to liquids and solids, though the calculations become more complex due to the stronger intermolecular forces and more restricted particle movement. In solids, the average kinetic energy is related to the vibrations of atoms around their equilibrium positions.
Conclusion: A Foundation of Physics and Chemistry
Average kinetic energy is a fundamental concept with far-reaching implications in physics and chemistry. Its connection to temperature, molecular motion, and chemical reactions is critical for understanding various physical and chemical phenomena. From the behavior of ideal gases to the mechanisms of chemical reactions, the concept of average kinetic energy provides a powerful tool for analyzing and predicting the behavior of matter at a macroscopic and microscopic level. Further exploration into statistical mechanics and thermodynamics will provide a deeper appreciation for the significance of this pivotal concept. Understanding average kinetic energy provides a foundation for further study in fields like materials science, astrophysics, and even engineering applications involving heat transfer and fluid dynamics.
Latest Posts
Latest Posts
-
How To Find All Zeros Of A Polynomial
Mar 26, 2025
-
How Do You Calculate The Heat Capacity Of A Calorimeter
Mar 26, 2025
-
Mendels Dihybrid Crosses Supported The Independent Hypothesis
Mar 26, 2025
-
Is Kinetic Energy Conserved In An Elastic Collision
Mar 26, 2025
-
Surplus And Shortage On A Graph
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Average Kinetic Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
