How Do You Calculate The Heat Capacity Of A Calorimeter
Muz Play
Mar 26, 2025 · 6 min read
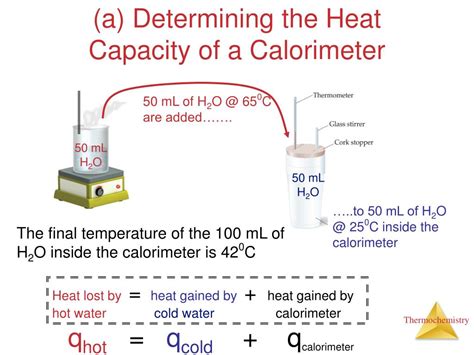
Table of Contents
How to Calculate the Heat Capacity of a Calorimeter
Determining the heat capacity of a calorimeter, often referred to as the calorimeter constant, is a crucial step in calorimetry experiments. This value represents the amount of heat required to raise the calorimeter's temperature by one degree Celsius (or one Kelvin). An accurately determined heat capacity is essential for precise measurements of enthalpy changes in chemical or physical processes. This comprehensive guide will walk you through the process, explaining the underlying principles, practical techniques, and calculations involved.
Understanding Heat Capacity and Calorimetry
Before delving into the calculations, let's establish a firm understanding of the fundamental concepts.
What is Heat Capacity?
Heat capacity (C) is a physical property that describes the amount of heat energy (q) needed to change the temperature (ΔT) of a substance by one degree. It's defined by the equation:
C = q / ΔT
The units of heat capacity are typically J/°C or J/K. It's important to note that the heat capacity of a substance can vary slightly depending on temperature and pressure.
Types of Calorimeters
Several types of calorimeters exist, each designed for specific applications. Common types include:
- Coffee-cup calorimeter: A simple, inexpensive calorimeter often used for determining the heat of reaction in solution. It consists of two nested Styrofoam cups with a lid.
- Bomb calorimeter (constant-volume calorimeter): Used for measuring the heat of combustion of substances, particularly fuels. Reactions occur in a sealed, high-pressure chamber.
- Differential scanning calorimeter (DSC): A more sophisticated instrument that measures the heat flow associated with phase transitions and other thermal events.
The method for determining the heat capacity differs slightly depending on the calorimeter type, but the underlying principles remain consistent.
Determining the Heat Capacity: The Method of Mixtures
The most common method for determining the heat capacity of a calorimeter is the method of mixtures. This technique involves adding a known amount of heat to the calorimeter and measuring the resulting temperature change. The heat capacity is then calculated using the following principles:
- Heat lost by the hot object = Heat gained by the calorimeter + Heat gained by the water (or other substance) inside the calorimeter
This principle relies on the conservation of energy: heat is neither created nor destroyed, only transferred.
Step-by-Step Procedure:
-
Prepare the calorimeter: Clean and dry the calorimeter thoroughly. If using a coffee-cup calorimeter, ensure the cups are tightly nested.
-
Measure the initial temperature: Record the initial temperature (T<sub>initial</sub>) of the calorimeter and its contents (typically water) with a precise thermometer. Ensure the thermometer is properly calibrated.
-
Add a known amount of heat: This is typically achieved by adding a known mass of a hot object (e.g., metal block) with a known specific heat capacity to the calorimeter. The hot object's initial temperature (T<sub>hot,initial</sub>) must be accurately measured before it's added.
-
Measure the final temperature: After adding the hot object, gently stir the mixture and monitor the temperature until it reaches a stable maximum temperature (T<sub>final</sub>). Record this final temperature.
-
Calculate the heat lost by the hot object: Use the following equation:
q<sub>hot</sub> = m<sub>hot</sub> * c<sub>hot</sub> * ΔT<sub>hot</sub>
where:
- q<sub>hot</sub> is the heat lost by the hot object (in Joules).
- m<sub>hot</sub> is the mass of the hot object (in grams).
- c<sub>hot</sub> is the specific heat capacity of the hot object (in J/g°C).
- ΔT<sub>hot</sub> = T<sub>hot,initial</sub> - T<sub>final</sub> (change in temperature of the hot object).
-
Calculate the heat gained by the water: Use the same equation as above, but with values for the water:
q<sub>water</sub> = m<sub>water</sub> * c<sub>water</sub> * ΔT<sub>water</sub>
where:
- q<sub>water</sub> is the heat gained by the water (in Joules).
- m<sub>water</sub> is the mass of the water (in grams).
- c<sub>water</sub> is the specific heat capacity of water (approximately 4.18 J/g°C).
- ΔT<sub>water</sub> = T<sub>final</sub> - T<sub>initial</sub> (change in temperature of the water).
-
Calculate the heat gained by the calorimeter: Since the heat lost by the hot object equals the heat gained by the calorimeter and water, we can find the heat gained by the calorimeter using:
q<sub>calorimeter</sub> = q<sub>hot</sub> - q<sub>water</sub>
-
Calculate the heat capacity of the calorimeter: Use the following equation:
C<sub>calorimeter</sub> = q<sub>calorimeter</sub> / ΔT<sub>water</sub>
where:
- C<sub>calorimeter</sub> is the heat capacity of the calorimeter (in J/°C).
- q<sub>calorimeter</sub> is the heat gained by the calorimeter (in Joules).
- ΔT<sub>water</sub> = T<sub>final</sub> - T<sub>initial</sub> (change in temperature of the water).
Factors Affecting Accuracy
Several factors can influence the accuracy of the heat capacity determination:
-
Heat loss to the surroundings: The calorimeter should be well-insulated to minimize heat exchange with the environment. Using a well-insulated calorimeter, like a bomb calorimeter, is crucial for accurate results. However, even in well-insulated calorimeters, heat loss will occur, often mitigated via statistical methods like extrapolation of temperature vs time graphs.
-
Incomplete mixing: Ensure thorough mixing of the contents of the calorimeter to ensure uniform temperature distribution.
-
Errors in temperature measurement: Using a properly calibrated thermometer with sufficient precision is essential. Multiple temperature readings are usually made and averaged.
-
Specific heat capacity of the materials: The accuracy of the specific heat capacities (c<sub>hot</sub> and c<sub>water</sub>) used in calculations affects the final result. The values should be obtained from reliable sources.
-
Calibration error of the calorimeter: Regular calibrations may be necessary to ensure accuracy. The procedure is usually performed at several temperatures, providing a temperature-dependent function of the calorimeter's constant.
Advanced Techniques and Considerations
For more sophisticated calorimetry experiments, more advanced techniques and considerations may be necessary. These include:
-
Correction for heat loss: Employing correction methods, such as the Regnault-Pfaundler method, which uses temperature readings taken over time before and after the reaction, to account for heat loss to the surrounding environment.
-
Using different reference materials: Rather than just a metal block, using a material with a precisely known heat of reaction can improve the accuracy and reliability of heat capacity calculations.
-
Utilizing specialized software: Data acquisition systems and analysis software are common in modern calorimetry, particularly with bomb calorimeters, providing more precise measurements and reducing calculation errors.
-
Accounting for the heat capacity of the stirrer: In some experiments, the heat capacity of the stirring mechanism needs to be considered.
-
Considering the heat capacity of the thermometer: A high-precision thermometer can have a noticeable impact on the final heat capacity value.
Conclusion
Accurately determining the heat capacity of a calorimeter is fundamental to performing reliable calorimetry experiments. The method of mixtures provides a straightforward approach, but careful attention to detail and consideration of potential sources of error are crucial for obtaining precise results. Understanding the underlying principles of heat transfer and employing appropriate techniques are key to ensuring the accuracy and reliability of calorimetric measurements. By meticulously following the procedures outlined above and considering the factors influencing accuracy, researchers can obtain accurate calorimeter constants, thus improving their calorimetry experiments' precision and results. The precision of heat capacity measurements directly influences the reliability of enthalpy calculations for chemical and physical processes.
Latest Posts
Latest Posts
-
Why Are Hydrogen Bonds Important For Life
Mar 29, 2025
-
Amount Of Lime To Neutralie 9 Lbs Of Solfuric Acid
Mar 29, 2025
-
Why Are Base Pairing Rules Important
Mar 29, 2025
-
Use The Cofactor Expansion To Compute The Following Determinant
Mar 29, 2025
-
How To Find Point Of Tangency
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Do You Calculate The Heat Capacity Of A Calorimeter . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
