What Happens When An Atom Gains An Electron
Muz Play
Mar 25, 2025 · 6 min read
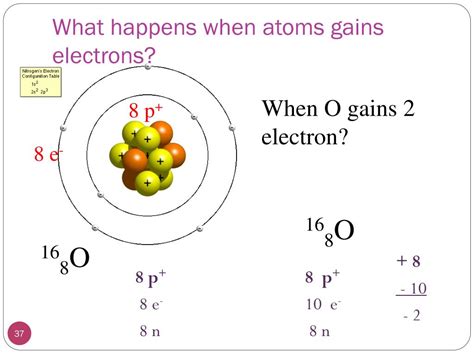
Table of Contents
What Happens When an Atom Gains an Electron? Delving into Ionization and its Effects
Atoms, the fundamental building blocks of matter, are typically depicted as neutral entities, possessing an equal number of positively charged protons in their nucleus and negatively charged electrons orbiting around it. However, this neutrality is not always the case. Atoms can gain or lose electrons, leading to a profound shift in their properties and behavior. This article explores the consequences of an atom gaining an electron, a process intimately tied to the formation of ions and the driving force behind various chemical and physical phenomena.
Understanding Atomic Structure and Electron Configuration
Before diving into the effects of electron gain, let's briefly revisit the basics of atomic structure. An atom's identity is determined by its atomic number, which represents the number of protons in its nucleus. Electrons reside in energy levels or shells surrounding the nucleus. These shells can hold a specific number of electrons; the first shell holds up to two, the second up to eight, and so on. The arrangement of electrons in these shells is known as the electron configuration, and it dictates an atom's chemical reactivity. Atoms strive for a stable electron configuration, usually a full outermost shell, a state often referred to as the octet rule (though exceptions exist).
The Process of Electron Gain: Forming Anions
When an atom gains an electron, it acquires a negative charge. This process is known as reduction, and the resulting negatively charged atom is called an anion. The electron is typically added to the outermost shell, making the atom more stable. This stability is achieved by filling the outermost shell closer to its maximum capacity, thereby reducing the atom's overall energy. The strength of this attraction to the added electron, and therefore the likelihood of an atom gaining an electron, is significantly influenced by the atom's electronegativity.
Electronegativity and its Role
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Highly electronegative atoms, such as those in Group 17 (halogens), have a strong tendency to gain an electron to achieve a stable electron configuration. On the other hand, atoms with low electronegativity, like alkali metals in Group 1, are more likely to lose electrons.
The Impact of Electron Gain: Changes in Properties
The addition of an electron dramatically alters an atom's properties. Here's a detailed breakdown of the key changes:
1. Change in Charge: From Neutral to Negative
The most immediate consequence is a change in the atom's net charge. The addition of a negatively charged electron results in a net negative charge, transforming a neutral atom into a negatively charged anion. This change in charge drastically alters the atom's interactions with other atoms and molecules.
2. Alteration of Chemical Reactivity: Increased Stability
Gaining an electron often leads to increased stability. Atoms gain electrons to achieve a more stable electron configuration, usually a full outer electron shell. This makes them less reactive compared to their neutral counterparts. However, it's important to note that even though they are more stable, they can still participate in chemical reactions – but their reactivity will differ from the neutral atom.
3. Modification of Physical Properties: Size and Density Changes
The addition of an electron also affects the atom's physical properties. The increase in the number of electrons leads to an increase in electron-electron repulsion. This repulsion causes the electron cloud to expand, resulting in a larger atomic radius. The increased size will directly affect the density of the material composed of these atoms, decreasing the overall density.
4. Impact on Electronic Configuration: Filling Outer Shells
A crucial consequence is the change in the atom's electron configuration. The gained electron occupies an available orbital in the outermost shell. This alters the atom’s electronic structure, affecting its energy levels and influencing its spectroscopic properties. For example, the absorption and emission of light will change based on the newly formed electronic configuration.
5. Effects on Magnetic Properties: Potential for Paramagnetism or Diamagnetism
The electron configuration also impacts the atom's magnetic properties. If the outermost shell has unpaired electrons after the addition of an electron, the atom will exhibit paramagnetism, meaning it will be attracted to a magnetic field. However, if all the electrons are paired, the atom will show diamagnetism, meaning it will be weakly repelled by a magnetic field.
Examples of Electron Gain and Anion Formation
Many common chemical reactions involve electron gain. Let's examine some examples:
1. Formation of Sodium Chloride (NaCl)
The classic example is the formation of table salt (NaCl). Sodium (Na) readily loses one electron to become a positively charged ion (Na+), while chlorine (Cl) readily gains one electron to become a negatively charged ion (Cl-). The electrostatic attraction between these oppositely charged ions forms the ionic bond in sodium chloride.
2. Formation of Oxide Ions (O²⁻)
Oxygen (O) is highly electronegative and readily gains two electrons to form an oxide ion (O²⁻). This process is crucial in the formation of many metal oxides, for example, in the rusting of iron.
3. Formation of Halide Ions (F⁻, Cl⁻, Br⁻, I⁻)
Halogens (Group 17 elements) are highly reactive nonmetals that readily gain one electron to form halide ions (F⁻, Cl⁻, Br⁻, I⁻). Their high electronegativity facilitates this electron gain.
Implications and Applications
The process of an atom gaining an electron has far-reaching implications across various scientific disciplines:
1. Chemistry: Ionic Bonding and Compound Formation
Electron gain is fundamental to ionic bonding, a significant type of chemical bond that plays a crucial role in forming a wide variety of compounds. Many minerals, salts, and other inorganic compounds are held together by ionic bonds. Understanding this process is essential for predicting the properties and reactivity of these compounds.
2. Physics: Understanding Material Properties
The resulting changes in atomic size, charge, and electronic configuration influence the overall physical properties of materials, including their electrical conductivity, magnetic properties, and optical behavior.
3. Biology: Biological Processes and Enzyme Function
In biological systems, electron transfer reactions are central to many processes, including respiration and photosynthesis. Enzymes often facilitate electron transfer, influencing the rates of biochemical reactions.
4. Engineering: Materials Science and Nanotechnology
The ability to control the gain or loss of electrons in materials has significant implications for material science and nanotechnology. This knowledge helps engineer materials with specific properties and functionalities.
Conclusion: A Fundamental Process with Wide-Ranging Effects
The seemingly simple process of an atom gaining an electron has profound implications for the properties and behavior of matter. It is a fundamental concept in chemistry and physics, crucial for understanding the formation of ionic compounds, the behavior of materials, and biological processes. From the formation of table salt to the intricate workings of cellular respiration, electron gain is a fundamental process shaping our world. Understanding this process helps us unlock the secrets of the universe and provides the foundation for numerous technological advancements. The exploration of atomic interactions at this fundamental level continuously unveils new possibilities and drives innovation across diverse scientific fields.
Latest Posts
Latest Posts
-
How Do You Know If A Reaction Is Redox
Mar 26, 2025
-
Como Sacar El Diametro De Un Circulo
Mar 26, 2025
-
How To Find All Zeros Of A Polynomial
Mar 26, 2025
-
How Do You Calculate The Heat Capacity Of A Calorimeter
Mar 26, 2025
-
Mendels Dihybrid Crosses Supported The Independent Hypothesis
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Happens When An Atom Gains An Electron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
