What Are The Building Blocks Of Macromolecules
Muz Play
Mar 25, 2025 · 7 min read
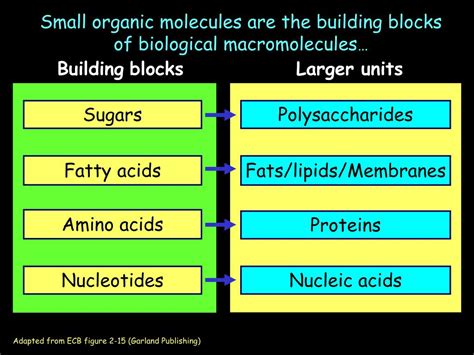
Table of Contents
What Are the Building Blocks of Macromolecules?
Macromolecules are giant molecules, essential for life, formed by the joining of smaller subunits called monomers. Understanding the building blocks of these macromolecules is fundamental to grasping the intricacies of biological processes. This comprehensive guide delves into the four major classes of macromolecules – carbohydrates, lipids, proteins, and nucleic acids – exploring their respective monomers and how these monomers combine to form the complex structures that underpin all living organisms.
Carbohydrates: The Body's Quick Energy Source
Carbohydrates, also known as saccharides, are the body's primary source of energy. They are composed of carbon, hydrogen, and oxygen atoms, typically in a ratio of 1:2:1. The basic building blocks of carbohydrates are monosaccharides, or simple sugars.
Monosaccharides: The Simple Sugars
Monosaccharides are the simplest form of carbohydrates and serve as the fundamental units for more complex carbohydrate structures. Key examples include:
- Glucose: A vital energy source for cells. It's found in fruits, honey, and corn syrup. Its linear and ring structures are crucial for understanding its role in metabolic pathways.
- Fructose: Found in fruits and honey, it's the sweetest of the monosaccharides. Its isomeric relationship with glucose highlights the subtle differences in molecular structure that can significantly impact function.
- Galactose: Less sweet than glucose or fructose, it's a component of lactose (milk sugar). Its role in lactose synthesis and its metabolic connection to glucose are important aspects to consider.
Disaccharides: Two Monosaccharides Joined
When two monosaccharides undergo a dehydration reaction, where a water molecule is removed, they form a disaccharide. This reaction forms a glycosidic bond. Common disaccharides include:
- Sucrose (table sugar): Composed of glucose and fructose.
- Lactose (milk sugar): Composed of glucose and galactose.
- Maltose (malt sugar): Composed of two glucose molecules.
Polysaccharides: Chains of Monosaccharides
Polysaccharides are long chains of monosaccharides linked by glycosidic bonds. They serve various functions, including energy storage and structural support. Examples include:
- Starch: A storage polysaccharide in plants, composed of amylose (a linear chain) and amylopectin (a branched chain) both made of glucose units. Understanding the branching patterns impacts its digestibility and energy release.
- Glycogen: The storage polysaccharide in animals, stored primarily in the liver and muscles. Its highly branched structure allows for rapid glucose release when needed.
- Cellulose: A structural polysaccharide in plant cell walls. Its linear structure and strong intermolecular forces make it incredibly strong and resistant to degradation. Humans cannot digest cellulose, unlike starch and glycogen, because of the specific beta-linkages in the cellulose molecule.
- Chitin: A structural polysaccharide found in the exoskeletons of arthropods and in the cell walls of fungi. Its unique properties provide strength and flexibility.
Lipids: Diverse Roles in Energy Storage and Structure
Lipids are a diverse group of hydrophobic (water-insoluble) molecules crucial for energy storage, cell membrane structure, and hormone signaling. Unlike carbohydrates, they don't have a common monomeric unit. However, many lipids are built from smaller subunits.
Fatty Acids: The Building Blocks of Many Lipids
Fatty acids are long hydrocarbon chains with a carboxyl group (-COOH) at one end. They are a key component of many lipids, including triglycerides and phospholipids. Fatty acids can be:
- Saturated: Have no double bonds between carbon atoms, making them relatively straight and tightly packed, often solid at room temperature.
- Unsaturated: Have one or more double bonds between carbon atoms, creating kinks in the chain and preventing tight packing, often liquid at room temperature. Unsaturated fatty acids can be further classified as monounsaturated (one double bond) or polyunsaturated (multiple double bonds).
Triglycerides: Energy Storage Powerhouses
Triglycerides are formed from the esterification of three fatty acids to a glycerol molecule. They are the primary form of energy storage in animals and plants. The type of fatty acids (saturated vs. unsaturated) affects the physical properties of triglycerides.
Phospholipids: Essential for Cell Membranes
Phospholipids are similar to triglycerides but have only two fatty acids attached to glycerol, with a phosphate group attached to the third carbon. This phosphate group is polar, making the molecule amphipathic – having both hydrophobic and hydrophilic regions. This property is crucial for their role in forming cell membranes.
Steroids: Diverse Roles in Cell Function
Steroids are characterized by a four-fused ring structure. Cholesterol is a crucial steroid component of cell membranes, and it serves as a precursor for the synthesis of steroid hormones like testosterone and estrogen. The complex ring structure is crucial to their biological activity.
Proteins: The Workhorses of the Cell
Proteins are incredibly diverse macromolecules performing a vast array of functions, including enzymatic catalysis, structural support, transport, and immune defense. Their building blocks are amino acids.
Amino Acids: The 20 Essential Building Blocks
Amino acids are composed of a central carbon atom bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and a variable side chain (R group). The R group determines the unique properties of each amino acid. There are 20 different amino acids commonly found in proteins. Understanding the properties of these side chains (polar, nonpolar, charged) is vital in predicting protein structure and function.
Peptide Bonds: Linking Amino Acids
Amino acids are linked together by peptide bonds to form polypeptide chains. A peptide bond is formed by a dehydration reaction between the carboxyl group of one amino acid and the amino group of another. The sequence of amino acids in a polypeptide chain determines the protein's primary structure.
Protein Structure: From Primary to Quaternary
Proteins have four levels of structural organization:
- Primary Structure: The linear sequence of amino acids.
- Secondary Structure: Local folding patterns, such as alpha-helices and beta-sheets, stabilized by hydrogen bonds between amino acid residues.
- Tertiary Structure: The overall three-dimensional arrangement of a polypeptide chain, stabilized by various interactions including hydrogen bonds, disulfide bridges, hydrophobic interactions, and ionic bonds.
- Quaternary Structure: The arrangement of multiple polypeptide chains (subunits) in a protein complex.
The protein's structure dictates its function. Changes in structure, often caused by alterations in amino acid sequence or environmental conditions (pH, temperature), can lead to protein denaturation and loss of function.
Nucleic Acids: The Carriers of Genetic Information
Nucleic acids, DNA (deoxyribonucleic acid) and RNA (ribonucleic acid), store and transmit genetic information. Their building blocks are nucleotides.
Nucleotides: The Monomers of Nucleic Acids
Nucleotides consist of three components:
- A pentose sugar: Ribose in RNA and deoxyribose in DNA.
- A phosphate group: Provides the backbone of the nucleic acid chain.
- A nitrogenous base: Adenine (A), guanine (G), cytosine (C), thymine (T) in DNA, and uracil (U) in RNA instead of thymine. These bases pair specifically (A with T/U, and G with C) through hydrogen bonding, forming the double helix structure of DNA.
Polynucleotide Chains: The Backbone of Nucleic Acids
Nucleotides are linked together by phosphodiester bonds between the phosphate group of one nucleotide and the sugar of the next, forming a polynucleotide chain. In DNA, two polynucleotide chains are twisted around each other to form the famous double helix structure. RNA is typically single-stranded, but can fold into complex three-dimensional structures.
The sequence of nucleotides in DNA and RNA encodes the genetic information that dictates the synthesis of proteins and other molecules. Understanding the base pairing rules and the structure of DNA and RNA is essential for comprehending inheritance, gene expression, and the regulation of cellular processes.
This comprehensive overview provides a foundational understanding of the building blocks of macromolecules. Remember that the structure and function of each macromolecule are intricately linked, and understanding their monomeric units and how they assemble into larger structures is key to understanding the complexity and beauty of biological systems. Further research into specific macromolecules and their roles in various biological processes can provide a deeper appreciation of their importance in life.
Latest Posts
Latest Posts
-
How To Determine If A Transformation Is Linear
Mar 26, 2025
-
How Do You Know If A Reaction Is Redox
Mar 26, 2025
-
Como Sacar El Diametro De Un Circulo
Mar 26, 2025
-
How To Find All Zeros Of A Polynomial
Mar 26, 2025
-
How Do You Calculate The Heat Capacity Of A Calorimeter
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Are The Building Blocks Of Macromolecules . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
