Is Table Salt A Mixture Or Pure Substance
Muz Play
Mar 25, 2025 · 5 min read
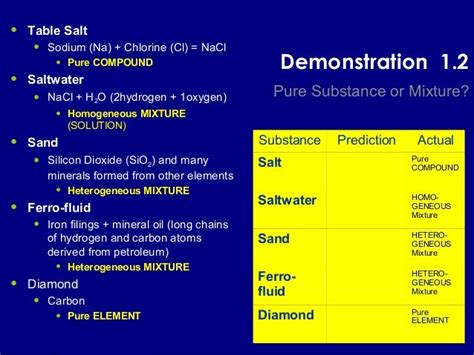
Table of Contents
Is Table Salt a Mixture or a Pure Substance? Delving into the Chemistry of NaCl
The seemingly simple question, "Is table salt a mixture or a pure substance?" opens a fascinating window into the world of chemistry and the intricacies of matter. While the answer might seem straightforward at first glance, a deeper exploration reveals nuances that enrich our understanding of chemical composition and the properties of substances. This comprehensive article will unravel the complexities of table salt, its composition, and its classification within the scientific framework of mixtures and pure substances.
Understanding Pure Substances and Mixtures
Before we delve into the specifics of table salt, let's establish a clear understanding of the fundamental terms: pure substances and mixtures.
Pure Substances: The Building Blocks of Matter
A pure substance is a form of matter that has a constant composition and properties throughout the sample. It cannot be separated into simpler substances by physical methods. Pure substances can be further categorized into:
-
Elements: These are the fundamental building blocks of matter, comprising only one type of atom. Examples include oxygen (O), iron (Fe), and gold (Au). Elements cannot be broken down into simpler substances through chemical reactions.
-
Compounds: These are substances formed by the chemical combination of two or more different elements in a fixed ratio. The properties of a compound are distinct from the properties of its constituent elements. Water (H₂O), carbon dioxide (CO₂), and table salt (NaCl) are examples of compounds. Compounds can be broken down into simpler substances through chemical reactions.
Mixtures: A Blend of Substances
A mixture is a combination of two or more substances that are physically combined but not chemically bonded. The components of a mixture retain their individual properties, and their proportions can vary. Mixtures can be further categorized into:
-
Homogeneous Mixtures: These have a uniform composition throughout the sample. The different components are not visibly distinguishable. Examples include saltwater, air, and sugar dissolved in water.
-
Heterogeneous Mixtures: These have a non-uniform composition. The different components are visibly distinguishable. Examples include sand and water, oil and water, and a salad.
The Composition of Table Salt: NaCl
Table salt, primarily known as sodium chloride (NaCl), is an ionic compound. This means it's formed through the electrostatic attraction between positively charged sodium ions (Na⁺) and negatively charged chloride ions (Cl⁻). These ions are held together by strong ionic bonds, a defining characteristic of a pure substance.
The Crystalline Structure of NaCl
NaCl exists in a highly ordered crystalline structure. Each sodium ion is surrounded by six chloride ions, and each chloride ion is surrounded by six sodium ions. This regular arrangement contributes to the salt's unique physical properties, such as its crystalline structure, high melting point, and solubility in water.
Purity vs. Practicality: Impurities in Table Salt
While chemically, pure NaCl is a pure substance, commercially available table salt usually contains additives. These additions are made to enhance its properties or to prevent caking. Common additives include:
- Iodine: Added to prevent iodine deficiency, a crucial nutrient for thyroid health.
- Anti-caking agents: Substances such as silicon dioxide or magnesium carbonate are added to prevent clumping due to moisture absorption.
These additions make table salt technically a mixture, albeit a mixture where the NaCl constitutes the vast majority of the sample. The added substances exist in small quantities and don't fundamentally alter the chemical nature of the salt.
Addressing the Question: Is Table Salt a Mixture or a Pure Substance?
The answer hinges on the level of detail being considered.
From a purely chemical perspective, pure sodium chloride (NaCl) is a pure substance – a compound. Its composition is fixed, and it has a unique set of chemical and physical properties. It is a chemical compound formed by the chemical bonding of sodium and chlorine.
However, commercially available table salt is a mixture. The addition of iodine, anti-caking agents, and potentially other trace minerals transforms the pure chemical compound into a complex mixture with various proportions of different components.
The Importance of Context: Pure Substances vs. Mixtures in Different Applications
The classification of table salt as a mixture or a pure substance depends heavily on the context. For chemical reactions and stoichiometric calculations, it's often treated as pure NaCl. In these scenarios, the minor additions are negligible and don't significantly influence the reaction or calculation.
On the other hand, in food science, nutrition, and industrial applications, the presence of added substances significantly impacts its properties and the overall quality of the product. In these instances, the classification of table salt as a mixture is more appropriate.
Exploring Further: Properties of Table Salt and its Components
Understanding the properties of both pure NaCl and the additives in table salt helps clarify its classification.
Properties of Sodium Chloride (NaCl):
- High melting point: Due to the strong ionic bonds.
- Crystalline structure: Forms cubic crystals.
- Solubility in water: Dissolves readily in water, forming an electrolyte solution.
- Taste: Characteristic salty flavor.
- White color: In its pure form.
Properties of Common Additives:
- Iodine: Essential micronutrient for thyroid function. It’s a non-metal element.
- Anti-caking agents (e.g., Silicon Dioxide): Inert substances that absorb moisture and prevent clumping. These are compounds.
The presence of these additives fundamentally alters some properties, such as the flowability and moisture absorption characteristics of the salt. The small quantities don't change the primary chemical nature, but significantly change the properties for everyday use and in manufacturing.
Conclusion: A Matter of Perspective
The question of whether table salt is a mixture or a pure substance illustrates the complex nature of chemical classification. While pure sodium chloride is undoubtedly a pure substance (a compound), commercially available table salt is more accurately classified as a mixture due to the inclusion of additives that alter its physical properties and enhance its usability. The best classification depends entirely on the context and the specific application being considered. This understanding highlights the importance of considering both the chemical composition and the practical applications when classifying a substance. Understanding this nuance further solidifies a comprehensive understanding of chemistry and its applications. The seemingly simple question of table salt's classification provides a gateway into the complex and fascinating world of chemical composition and the multifaceted nature of matter.
Latest Posts
Latest Posts
-
A Chemical Equation Is Balanced When
Mar 25, 2025
-
What Is The Average Kinetic Energy
Mar 25, 2025
-
What Are The Building Blocks Of Macromolecules
Mar 25, 2025
-
Chemistry The Molecular Nature Of Matter And Change 8th Edition
Mar 25, 2025
-
Which Event Always Involves A Chemical Change
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Is Table Salt A Mixture Or Pure Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
