Bohr Diagrams For The First 20 Elements
Muz Play
Mar 22, 2025 · 6 min read
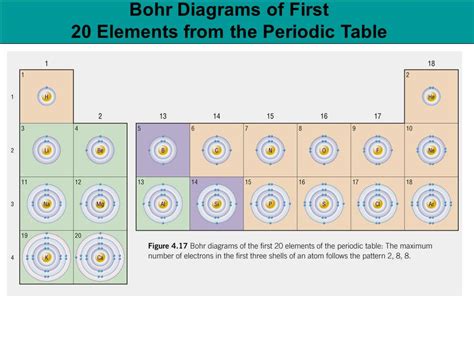
Table of Contents
Bohr Diagrams for the First 20 Elements: A Comprehensive Guide
Bohr diagrams, also known as Bohr models, are simplified representations of atomic structure. They provide a visual way to understand the arrangement of electrons in energy levels or shells surrounding an atom's nucleus. This is crucial for understanding an element's chemical properties and how it interacts with other elements. This comprehensive guide will explore Bohr diagrams for the first 20 elements of the periodic table, providing a detailed explanation of their structure and the principles behind their construction.
Understanding the Basics of Bohr Diagrams
Before diving into specific elements, let's review the fundamental components of a Bohr diagram:
- Nucleus: The central part of the atom, containing protons (positively charged) and neutrons (neutral). The number of protons determines the atomic number and identifies the element.
- Electron Shells/Energy Levels: Surrounding the nucleus are concentric circles representing energy levels or shells. Electrons, negatively charged particles, occupy these shells. Each shell has a maximum number of electrons it can hold.
- Electron Configuration: The arrangement of electrons in the shells dictates an element's chemical behavior.
The Shell Rules:
- Shell 1 (K shell): Holds a maximum of 2 electrons.
- Shell 2 (L shell): Holds a maximum of 8 electrons.
- Shell 3 (M shell): Holds a maximum of 18 electrons (but for the first 20 elements, it rarely exceeds 8).
- Shell 4 (N shell) and beyond: While these shells exist, they are not relevant for the first 20 elements.
Electrons fill the shells starting with the lowest energy level (closest to the nucleus) before moving to higher energy levels. This principle is essential for understanding the electron configuration.
Constructing Bohr Diagrams: A Step-by-Step Approach
To draw a Bohr diagram, follow these steps:
- Determine the Atomic Number: Find the atomic number of the element from the periodic table. This number represents the number of protons and, in a neutral atom, the number of electrons.
- Draw the Nucleus: Represent the nucleus as a circle in the center, and write the element's symbol and atomic number inside it (e.g., ₂He for Helium).
- Determine the Number of Electrons: The number of electrons is equal to the atomic number for neutral atoms.
- Fill the Electron Shells: Start filling electrons into the shells, beginning with the shell closest to the nucleus (Shell 1). Remember the maximum capacity of each shell.
- Represent Electrons: Draw electrons as dots or crosses around the nucleus in each shell.
Bohr Diagrams for the First 20 Elements
Let's now create Bohr diagrams for the first 20 elements, explaining the arrangement of electrons in each case:
1. Hydrogen (H, Atomic Number 1):
- Nucleus: ₁H
- Electrons: 1 electron in Shell 1
1e-
-------
| |
-------
₁H
2. Helium (He, Atomic Number 2):
- Nucleus: ₂He
- Electrons: 2 electrons in Shell 1 (Shell 1 is full)
2e-
-------
| |
-------
₂He
3. Lithium (Li, Atomic Number 3):
- Nucleus: ₃Li
- Electrons: 2 electrons in Shell 1, 1 electron in Shell 2
2e- 1e-
------- ---
| | | |
------- ---
₃Li
4. Beryllium (Be, Atomic Number 4):
- Nucleus: ₄Be
- Electrons: 2 electrons in Shell 1, 2 electrons in Shell 2
2e- 2e-
------- ---
| | | |
------- ---
₄Be
5. Boron (B, Atomic Number 5):
- Nucleus: ₅B
- Electrons: 2 electrons in Shell 1, 3 electrons in Shell 2
2e- 3e-
------- ---
| | | |
------- ---
₅B
6. Carbon (C, Atomic Number 6):
- Nucleus: ₆C
- Electrons: 2 electrons in Shell 1, 4 electrons in Shell 2
2e- 4e-
------- ---
| | | |
------- ---
₆C
7. Nitrogen (N, Atomic Number 7):
- Nucleus: ₇N
- Electrons: 2 electrons in Shell 1, 5 electrons in Shell 2
2e- 5e-
------- ---
| | | |
------- ---
₇N
8. Oxygen (O, Atomic Number 8):
- Nucleus: ₈O
- Electrons: 2 electrons in Shell 1, 6 electrons in Shell 2
2e- 6e-
------- ---
| | | |
------- ---
₈O
9. Fluorine (F, Atomic Number 9):
- Nucleus: ₉F
- Electrons: 2 electrons in Shell 1, 7 electrons in Shell 2
2e- 7e-
------- ---
| | | |
------- ---
₉F
10. Neon (Ne, Atomic Number 10):
- Nucleus:₁₀Ne
- Electrons: 2 electrons in Shell 1, 8 electrons in Shell 2 (Shell 2 is full)
2e- 8e-
------- ---
| | | |
------- ---
₁₀Ne
11. Sodium (Na, Atomic Number 11):
- Nucleus:₁₁Na
- Electrons: 2 electrons in Shell 1, 8 electrons in Shell 2, 1 electron in Shell 3
2e- 8e- 1e-
------- --- ---
| | | | | |
------- --- ---
₁₁Na
12-20: Continuing the Pattern
Following the same principles, we can construct Bohr diagrams for the remaining elements (Magnesium through Calcium). The pattern continues, with electrons filling the shells sequentially, following the maximum capacity of each shell. For elements beyond Neon, the third shell (M shell) begins to fill. Remember that although the M shell can hold up to 18 electrons, for the first 20 elements, it rarely exceeds 8 electrons before the next shell starts filling.
Significance of Bohr Diagrams in Chemistry
Bohr diagrams are essential tools for visualizing:
- Chemical Bonding: The outermost electrons (valence electrons) determine how an atom bonds with other atoms. Elements with similar numbers of valence electrons tend to exhibit similar chemical properties.
- Reactivity: Elements with incomplete outermost shells are generally more reactive than those with full outermost shells (like noble gases).
- Periodic Trends: Bohr diagrams help explain trends in the periodic table, such as electronegativity and ionization energy.
Limitations of Bohr Diagrams
While Bohr diagrams are valuable for visualizing atomic structure, they have limitations:
- Simplified Model: They don't accurately represent the complex behavior of electrons, such as their wave-particle duality and the shapes of their orbitals.
- Energy Levels: The diagram doesn't show the relative energy differences between subshells within a given shell.
- Quantum Mechanics: They don't reflect the complexities of quantum mechanics, which provides a more accurate description of atomic structure.
Despite these limitations, Bohr diagrams serve as a useful introductory tool for understanding fundamental concepts of atomic structure and chemical bonding for beginner chemistry students. They provide a simplified visual representation that is easy to grasp and lay the foundation for more advanced concepts.
Conclusion
Bohr diagrams, although a simplification, provide a fundamental understanding of atomic structure, especially for the first 20 elements. By visualizing the arrangement of electrons in energy levels, we can gain insights into the chemical behavior and reactivity of these elements. While more advanced models exist to describe atomic structure more accurately, Bohr diagrams remain a valuable tool for introductory chemistry education. Understanding how to construct and interpret these diagrams is crucial for building a solid foundation in chemistry. Remember to always practice and create your own diagrams to solidify your understanding of electron configurations.
Latest Posts
Latest Posts
-
Hydrogen Bonds Are Weak Or Strong
Mar 23, 2025
-
What Elements Are Most Likey To Becom Anions
Mar 23, 2025
-
What Organelles Are Found In Plant Cells Only
Mar 23, 2025
-
The Rate Constant For This First Order Reaction Is
Mar 23, 2025
-
Is Chemical Energy Potential Or Kinetic Energy
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Bohr Diagrams For The First 20 Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
