Hydrogen Bonds Are Weak Or Strong
Muz Play
Mar 23, 2025 · 6 min read
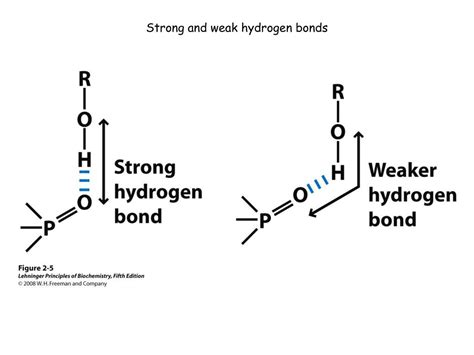
Table of Contents
Hydrogen Bonds: Weak or Strong? A Comprehensive Look
The question of whether hydrogen bonds are weak or strong is a complex one, with the answer depending heavily on context. While individually they are weaker than covalent or ionic bonds, their collective strength and prevalence in biological systems and materials science make them incredibly significant. This article delves deep into the nature of hydrogen bonds, exploring their strengths and weaknesses, their crucial roles in various fields, and the factors that influence their overall strength.
Understanding the Nature of Hydrogen Bonds
Hydrogen bonds are a special type of dipole-dipole attraction between molecules, not a true chemical bond. They arise when a hydrogen atom covalently bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) experiences attraction to another electronegative atom in a nearby molecule. This electronegativity difference creates a significant polarity, with the hydrogen atom carrying a partial positive charge (δ+) and the electronegative atom a partial negative charge (δ-). This electrostatic attraction forms the hydrogen bond.
Key Characteristics of Hydrogen Bonds:
- Strength: While weaker than covalent bonds, hydrogen bonds are significantly stronger than other intermolecular forces like van der Waals forces. Their strength typically falls in the range of 1-40 kJ/mol, significantly lower than the 150-800 kJ/mol range for covalent bonds.
- Directionality: Hydrogen bonds are highly directional, meaning they are strongest when the hydrogen atom, the electronegative atom it's bonded to, and the acceptor atom are in a linear arrangement. Deviations from this linearity weaken the bond.
- Length: The length of a hydrogen bond varies depending on the participating atoms and their environment, typically ranging from 1.5 to 2.6 Å (angstroms).
- Number: The overall strength of hydrogen bonding within a system isn't just determined by the strength of individual bonds but also by the number of bonds present. Many weak bonds collectively can exert a significant force.
Why the "Weak" and "Strong" Distinction is Relative
The apparent contradiction of hydrogen bonds being both "weak" and "strong" stems from the different scales of comparison and the context in which they're considered.
Hydrogen Bonds are Weak Compared To:
- Covalent Bonds: Covalent bonds involve the sharing of electron pairs between atoms, resulting in a much stronger and more stable interaction than the electrostatic attraction of hydrogen bonds. Covalent bonds are fundamental to the structure of molecules themselves, whereas hydrogen bonds exist between molecules or different parts of the same large molecule.
- Ionic Bonds: Ionic bonds arise from the electrostatic attraction between oppositely charged ions, forming strong crystalline structures. Hydrogen bonds, while electrostatic in nature, are much weaker because the charges involved are partial charges, not full charges.
Hydrogen Bonds are Strong Compared To:
- Van der Waals Forces: These forces are weak, short-range intermolecular attractions resulting from temporary fluctuations in electron distribution. Hydrogen bonds are substantially stronger than these forces due to the specific and directional nature of the interaction.
- Other Intermolecular Forces: Compared to other dipole-dipole interactions or London dispersion forces, hydrogen bonds exhibit considerably higher strength and specificity. This strength is crucial for the properties of many substances.
The Impact of Hydrogen Bonds Across Different Disciplines
The seemingly paradoxical nature of hydrogen bonds – weak individually but strong collectively – profoundly affects various fields:
1. Biochemistry and Biology: The Foundation of Life
Hydrogen bonds play an absolutely pivotal role in maintaining the structure and function of biological macromolecules:
- Proteins: The secondary, tertiary, and quaternary structures of proteins are largely stabilized by hydrogen bonds between amino acid residues. These bonds determine the protein's three-dimensional shape, which is essential for its biological activity. Alpha-helices and beta-sheets, crucial secondary structures, are stabilized by extensive networks of hydrogen bonds.
- Nucleic Acids (DNA and RNA): The double helix structure of DNA is maintained by hydrogen bonds between complementary base pairs (adenine with thymine, and guanine with cytosine). These bonds are essential for storing and transmitting genetic information. The specificity of base pairing relies on the precise geometry and strength of these hydrogen bonds.
- Water: Water's unique properties—high boiling point, high surface tension, excellent solvent—are largely attributed to its extensive hydrogen bonding network. Each water molecule can form up to four hydrogen bonds with neighboring molecules, creating a highly structured and cohesive liquid. This is crucial for life as we know it.
- Enzyme-Substrate Interactions: Hydrogen bonds often contribute to the specific binding between enzymes and their substrates, facilitating enzymatic reactions.
2. Materials Science: Designing with Hydrogen Bonds
The strength and directionality of hydrogen bonds are exploited in materials science to design materials with specific properties:
- Polymer Science: Hydrogen bonding influences the properties of polymers, such as their strength, flexibility, and melting points. Polymers with extensive hydrogen bonding tend to be stronger and have higher melting points.
- Crystal Engineering: Hydrogen bonds are used to create supramolecular structures and crystals with predetermined architectures. The precise control over hydrogen bond formation allows the design of materials with tailored optical, electronic, and mechanical properties.
- Self-Assembly: Hydrogen bonds play a crucial role in self-assembling systems, where molecules spontaneously organize themselves into complex structures. This is being explored in the development of nanomaterials and drug delivery systems.
3. Environmental Science: Water and Climate
Hydrogen bonding in water plays a crucial role in various environmental processes:
- Water Cycle: The high heat capacity of water due to hydrogen bonding moderates temperature fluctuations on Earth, influencing weather patterns and climate.
- Water's Role as a Solvent: Water's ability to dissolve numerous substances due to its polar nature and hydrogen bonding capacity is essential for various environmental processes and biological systems.
Factors Affecting Hydrogen Bond Strength
Several factors can influence the strength of hydrogen bonds:
- Electronegativity: The greater the electronegativity difference between the hydrogen atom's donor atom and the acceptor atom, the stronger the hydrogen bond. O-H···O bonds are generally stronger than N-H···N bonds.
- Distance: Shorter hydrogen bond lengths generally correspond to stronger bonds. However, excessively short distances can lead to repulsive interactions, weakening the bond.
- Linearity: The more linear the arrangement of the donor atom, hydrogen atom, and acceptor atom, the stronger the bond. Deviations from linearity reduce bond strength due to less efficient charge overlap.
- Solvent Effects: The surrounding solvent can affect hydrogen bond strength by competing for hydrogen bonds or by altering the dielectric constant of the environment.
- Temperature and Pressure: Higher temperatures weaken hydrogen bonds, while higher pressures can strengthen them, especially in dense systems.
Conclusion: The Importance of Context
The question of whether hydrogen bonds are weak or strong depends entirely on the context. Compared to covalent and ionic bonds, they are relatively weak. However, their collective strength, directionality, and prevalence in biological and materials science systems make them incredibly important. Understanding the multifaceted nature of hydrogen bonds is critical for advancing our knowledge in diverse scientific disciplines, from biology and chemistry to materials science and environmental science. Their subtle yet powerful influence shapes the world around us, influencing everything from the structure of DNA to the properties of the materials we use every day. Further research into the nuanced aspects of hydrogen bonding continues to reveal its far-reaching implications and potential for innovation.
Latest Posts
Latest Posts
-
Choose The Thermodynamic Product Formed During The Reaction Depicted Below
Mar 25, 2025
-
General Chemistry Principles And Modern Applications By Petrucci
Mar 25, 2025
-
Environmental Factors That Influence Microbial Growth
Mar 25, 2025
-
Electrons In An Atoms Outermost Energy Shells Are Called
Mar 25, 2025
-
Which Kingdoms Contain Organisms That Are Prokaryotes
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Hydrogen Bonds Are Weak Or Strong . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
