Choose The Thermodynamic Product Formed During The Reaction Depicted Below
Muz Play
Mar 25, 2025 · 5 min read
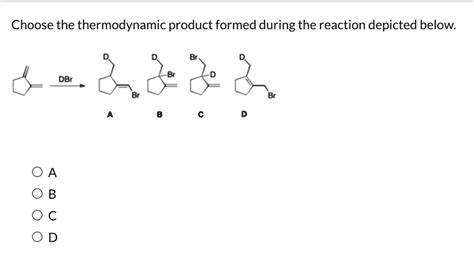
Table of Contents
Choosing the Thermodynamic Product: A Deep Dive into Reaction Outcomes
Understanding reaction outcomes is crucial in organic chemistry. Often, reactions can yield multiple products, and predicting which product will be the major one requires a thorough understanding of thermodynamics and kinetics. This article delves into the factors determining the thermodynamic product, focusing on how to identify it in a given reaction scenario. We will explore various reaction types and the influence of reaction conditions on the product distribution.
What are Kinetic and Thermodynamic Products?
Before we can choose the thermodynamic product, we need a clear definition. Reactions often proceed through multiple pathways, each leading to a different product. These products are classified as either kinetic or thermodynamic products based on their formation pathways and relative stabilities.
-
Kinetic Product: This product is formed faster, often at lower temperatures. It's favored by the lower activation energy of its formation pathway, even if it's less stable than other possible products. The reaction is under kinetic control.
-
Thermodynamic Product: This product is the most stable product. It's favored at higher temperatures and longer reaction times, as the reaction has sufficient time to overcome activation energy barriers and reach equilibrium. The reaction is under thermodynamic control.
Think of it like this: Imagine you have two hills. One is short and steep (kinetic product), the other is tall and gradual (thermodynamic product). To get to the top of the short hill is quick (fast reaction), but the top is not as high (less stable). The tall hill requires more effort (higher activation energy), but the summit is much higher (more stable). The reaction conditions determine which hill you climb.
Factors Influencing Thermodynamic Product Formation
Several factors influence which product is favored – the kinetic or the thermodynamic one:
-
Temperature: Higher temperatures favor the thermodynamic product. At higher temperatures, molecules possess more energy to overcome higher activation barriers, allowing the reaction to proceed towards the more stable product.
-
Reaction Time: Longer reaction times also favor the thermodynamic product. Sufficient time allows the reaction to reach equilibrium, where the most stable product is predominantly formed.
-
Catalyst: The presence of a catalyst can affect the activation energy of different pathways, potentially altering the product distribution. Catalysts speed up reactions, and if they preferentially lower the activation energy for the thermodynamic product pathway, they can increase its yield.
-
Solvent: The solvent used in the reaction can influence the stability of the reactants and products, consequently affecting the product distribution. Polar solvents often stabilize polar molecules, while nonpolar solvents stabilize nonpolar molecules.
-
Reactant Concentration: The concentration of the reactants can also have an impact. High concentrations may accelerate reactions that lead to the kinetic product, while lower concentrations may give the thermodynamic product more time to form.
Identifying the Thermodynamic Product in a Reaction
Identifying the thermodynamic product requires analyzing the stability of the potential products. Generally, the following factors contribute to greater stability:
-
Resonance Stabilization: Molecules with extended pi systems (delocalized electrons) are more stable due to resonance stabilization. The more resonance structures a molecule possesses, the more stable it is likely to be.
-
Hyperconjugation: The interaction between electrons in a sigma bond (typically C-H) and an adjacent empty p-orbital or partially filled p-orbital (like a carbocation) increases stability through hyperconjugation. More hyperconjugative interactions lead to increased stability.
-
Steric Hindrance: Bulky groups around a molecule lead to steric hindrance, destabilizing the molecule. Less steric hindrance means greater stability.
-
Inductive Effects: Electron-donating or electron-withdrawing groups can influence the stability of a molecule through inductive effects. Electron-donating groups stabilize positively charged molecules, while electron-withdrawing groups stabilize negatively charged molecules.
-
Bond Strengths: Stronger bonds lead to greater stability. For example, a C=C double bond is stronger than a C-C single bond.
Example: Consider the addition of HBr to 1,3-butadiene. Two products are possible: 1,2-addition and 1,4-addition. At lower temperatures, the kinetic product (1,2-addition) is favored. However, at higher temperatures, the thermodynamic product (1,4-addition) predominates because the 1,4-addition product is more stable due to greater conjugation and resonance stabilization. The more substituted double bond is energetically favored.
Practical Applications and Significance
The ability to predict and control the formation of thermodynamic products has significant implications in various fields:
-
Drug Discovery: In the pharmaceutical industry, controlling the formation of specific isomers (different spatial arrangements of atoms) is crucial. Often, only one isomer of a drug molecule possesses the desired biological activity. Understanding thermodynamic control enables the synthesis of the desired isomer.
-
Polymer Chemistry: The properties of polymers depend heavily on the structure of their monomers and the way they are linked. Thermodynamic control allows the creation of polymers with specific properties.
-
Materials Science: The design and synthesis of new materials often involve controlling the formation of specific crystalline structures. Understanding thermodynamic principles enables the development of materials with tailored properties.
Conclusion
Determining the thermodynamic product in a reaction requires a careful consideration of several factors, including temperature, reaction time, catalyst, solvent, and reactant concentrations. By analyzing the relative stability of potential products based on resonance, hyperconjugation, steric hindrance, inductive effects, and bond strengths, chemists can predict and control the outcome of reactions, leading to significant advancements in various scientific and technological fields. The choice between kinetic and thermodynamic control is crucial in directing the outcome of a chemical reaction and shaping the properties of the resulting products. A deep understanding of these principles is vital for synthetic chemists and materials scientists alike. Further research into reaction mechanisms and the influence of reaction conditions continues to refine our ability to predict and manipulate reaction outcomes with precision. The ongoing development of new catalysts and synthetic methodologies is further pushing the boundaries of chemical synthesis, allowing access to an even broader range of products with tailored properties.
Latest Posts
Latest Posts
-
What Is The Instrument To Measure Humidity
Mar 25, 2025
-
El Preterito De Los Verbos Regulares
Mar 25, 2025
-
Do Transition Metals Have A Charge
Mar 25, 2025
-
How To Calculate The Potential Difference
Mar 25, 2025
-
Why Race Is A Social Construct
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Choose The Thermodynamic Product Formed During The Reaction Depicted Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
