What Elements Are Most Likey To Becom Anions
Muz Play
Mar 23, 2025 · 5 min read
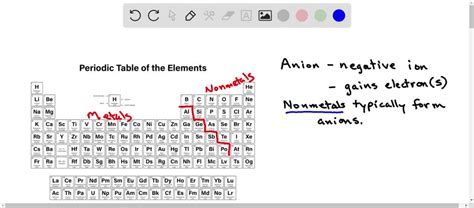
Table of Contents
What Elements Are Most Likely to Become Anions?
Understanding the formation of anions—negatively charged ions—is fundamental to grasping chemical bonding and reactivity. Anions form when an atom gains one or more electrons, achieving a more stable electron configuration, often resembling that of a noble gas. This article delves into the elements most likely to form anions, exploring their electronic structure, periodic trends, and the factors influencing their anionic behavior.
The Electronegativity Factor: A Key Determinant
The propensity of an element to become an anion is strongly correlated with its electronegativity. Electronegativity is a measure of an atom's ability to attract electrons towards itself within a chemical bond. Elements with high electronegativity have a stronger pull on electrons, making them more likely to gain electrons and form anions.
Periodic Trends in Electronegativity
Electronegativity generally increases across a period (from left to right) on the periodic table and decreases down a group (from top to bottom). This means that elements located in the upper right-hand corner of the periodic table, excluding noble gases, tend to exhibit the highest electronegativities and are therefore the most likely to form anions.
Nonmetals: The Anion Champions
Nonmetals, located on the right side of the periodic table, are renowned for their ability to readily gain electrons and form anions. This is because their valence shells (outermost electron shells) are relatively close to being filled. Gaining a few electrons completes their valence shell, achieving the stable electron configuration of a noble gas, a state of low energy and high stability.
-
Halogens (Group 17): The halogens (fluorine, chlorine, bromine, iodine, and astatine) are exceptionally electronegative and readily form anions with a -1 charge (e.g., F⁻, Cl⁻, Br⁻, I⁻). Their high electronegativity stems from their seven valence electrons, requiring only one additional electron to achieve a stable octet.
-
Chalcogens (Group 16): Chalcogens (oxygen, sulfur, selenium, tellurium, and polonium) are also highly electronegative, although less so than halogens. They typically form anions with a -2 charge (e.g., O²⁻, S²⁻, Se²⁻). This is because they have six valence electrons and require two more to achieve a stable octet.
-
Nitrogen (Group 15): Nitrogen, while less electronegative than halogens and chalcogens, can also form anions, although less readily. It commonly forms a nitride anion (N³⁻) in certain compounds.
Metals: The Anion Exceptions
Metals, generally located on the left side of the periodic table, are characterized by their low electronegativity and tendency to lose electrons rather than gain them. This means they usually form cations (positively charged ions) rather than anions. However, there are exceptions.
Transition Metals and Anion Formation
Some transition metals, particularly those in higher oxidation states, can exhibit behavior that leads to anion formation under specific conditions. These conditions usually involve the presence of strongly electronegative ligands or specific reaction environments that stabilize the anionic state. For example, certain complex ions involving transition metals can possess a negative charge. This is less about the inherent tendency of the metal to gain electrons and more about the overall charge balance of the complex ion.
Metalloids: A Gray Area
Metalloids, situated between metals and nonmetals on the periodic table (e.g., boron, silicon, arsenic), exhibit intermediate electronegativity values. Their behavior can vary depending on the chemical environment. Under certain circumstances, some metalloids can form anions, but this is less common than with nonmetals.
Factors Influencing Anion Formation Beyond Electronegativity
While electronegativity plays a dominant role, other factors influence the likelihood of anion formation:
-
Ionization Energy: The energy required to remove an electron from an atom. Elements with high ionization energies are less likely to lose electrons, making it more favorable for them to gain electrons and form anions. This complements the electronegativity factor.
-
Electron Affinity: The energy change that occurs when an atom gains an electron. A high electron affinity indicates a strong tendency to gain electrons, further enhancing the likelihood of anion formation.
-
Size of the Atom: Larger atoms tend to have lower electronegativities because the outermost electrons are farther from the nucleus and experience less attractive force. However, their larger size allows for better accommodation of additional electrons without significant repulsion, which can influence anion stability.
-
Chemical Environment: The surrounding atoms and molecules in a chemical reaction can significantly influence the stability and formation of anions. Certain chemical environments can stabilize an otherwise unstable anionic state.
Examples of Common Anions
- Halide ions: F⁻, Cl⁻, Br⁻, I⁻
- Oxide ion: O²⁻
- Sulfide ion: S²⁻
- Nitride ion: N³⁻
- Phosphate ion: PO₄³⁻
- Sulfate ion: SO₄²⁻
- Nitrate ion: NO₃⁻
- Carbonate ion: CO₃²⁻
- Hydroxide ion: OH⁻
Practical Applications and Significance of Anions
Understanding anion formation is crucial in various fields:
- Chemistry: Predicting reactivity, understanding chemical bonding, and designing new materials.
- Biology: Anions play critical roles in biological processes, such as nerve impulse transmission (chloride ions), and maintaining pH balance (bicarbonate ions).
- Materials Science: Designing materials with specific electronic and optical properties.
- Environmental Science: Understanding pollutant behavior and remediation strategies.
Conclusion: Predicting Anion Formation
While electronegativity is the primary factor determining an element's propensity to form anions, other factors, such as ionization energy, electron affinity, atomic size, and the chemical environment, play significant roles. Elements with high electronegativity, particularly nonmetals such as halogens and chalcogens, are most likely to gain electrons and form stable anions. However, exceptions exist, and the overall stability of the resulting anion within a specific chemical context is crucial to its formation. This comprehensive understanding of anion formation is pivotal in various scientific disciplines and technological advancements.
Latest Posts
Latest Posts
-
Electrons In An Atoms Outermost Energy Shells Are Called
Mar 25, 2025
-
Which Kingdoms Contain Organisms That Are Prokaryotes
Mar 25, 2025
-
What Element Is Found In Proteins
Mar 25, 2025
-
2 Sample Z Test For Proportions
Mar 25, 2025
-
Last Of Five Rhyming Greek Letters
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Elements Are Most Likey To Becom Anions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
