What Element Is Found In Proteins
Muz Play
Mar 25, 2025 · 6 min read
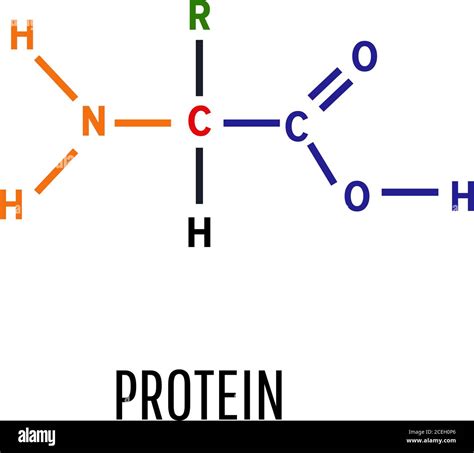
Table of Contents
What Element is Found in Proteins? A Deep Dive into the Building Blocks of Life
Proteins are the workhorses of the cell, involved in virtually every biological process imaginable. From catalyzing reactions as enzymes to providing structural support, their functions are incredibly diverse. But what makes these remarkable molecules tick? The answer lies in their fundamental composition: proteins are primarily composed of carbon (C), hydrogen (H), oxygen (O), and nitrogen (N). While these four elements are crucial, other elements, such as sulfur (S) and phosphorus (P), also play significant roles in certain proteins. This article will delve into the specifics of these elements, their contributions to protein structure and function, and the consequences of their absence or alteration.
The Big Four: Carbon, Hydrogen, Oxygen, and Nitrogen
The backbone of every protein is formed by a chain of amino acids. Each amino acid contains a central carbon atom, known as the alpha carbon, bonded to four different groups:
-
Carboxyl group (-COOH): This acidic group contributes to the overall charge of the amino acid and participates in peptide bond formation. The oxygen atoms within this group are essential for its functionality.
-
Amino group (-NH2): This basic group also participates in peptide bond formation and plays a crucial role in determining the amino acid's charge at different pH levels. The nitrogen atom is absolutely critical here.
-
Hydrogen atom (-H): While seemingly simple, the hydrogen atom contributes significantly to the overall polarity and hydrogen bonding capabilities of the amino acid.
-
Side chain (R group): This variable group is what differentiates one amino acid from another and dictates its unique properties (size, charge, polarity, etc.). The composition of the R group can include carbon, hydrogen, oxygen, nitrogen, and sulfur.
The peptide bond, which links amino acids together to form a polypeptide chain, is formed between the carboxyl group of one amino acid and the amino group of the next. This bond involves a carbon atom from the carboxyl group, a nitrogen atom from the amino group, and oxygen and hydrogen atoms. The precise arrangement of these atoms within the peptide bond leads to a planar structure that influences the overall protein conformation.
The Role of Each Element:
-
Carbon (C): Carbon forms the structural backbone of the amino acids and the peptide bonds. Its ability to form four covalent bonds allows for the creation of complex and diverse amino acid structures.
-
Hydrogen (H): Hydrogen atoms are abundant in proteins, participating in various non-covalent interactions, such as hydrogen bonds. These interactions are crucial for maintaining the three-dimensional structure of proteins.
-
Oxygen (O): Oxygen is found in the carboxyl group of amino acids, where it plays a crucial role in both peptide bond formation and the acidic properties of the group. Oxygen is also present in many side chains of various amino acids, affecting their polarity and interactions.
-
Nitrogen (N): Nitrogen is a crucial element found in the amino group of amino acids and in the side chains of several amino acids. Its presence is essential for the formation of the peptide bond and for the overall functionality of many amino acids. The nitrogen atoms often participate in hydrogen bonds and other crucial interactions that shape the protein.
Beyond the Big Four: Sulfur and Phosphorus
While carbon, hydrogen, oxygen, and nitrogen form the foundation of all proteins, some proteins also incorporate other elements.
Sulfur (S):
Sulfur is found in the side chains of two amino acids: cysteine and methionine.
-
Cysteine: The sulfur atom in cysteine is crucial for the formation of disulfide bonds. These strong covalent bonds link together cysteine residues in different parts of the polypeptide chain, stabilizing the protein's three-dimensional structure. Disulfide bonds are particularly important in extracellular proteins, which are exposed to harsher conditions.
-
Methionine: While methionine's sulfur atom doesn't participate in bond formation in the same way as cysteine, it plays a vital role in initiating protein synthesis and in several metabolic pathways. Its unique properties contribute to the overall functionality of proteins.
Phosphorus (P):
Phosphorus is less common in protein structure compared to sulfur, but it plays a crucial role in some proteins. It is often found in the form of phosphoryl groups, which can be added to or removed from certain amino acids through a process called phosphorylation. Phosphorylation is a crucial mechanism for regulating protein activity, acting as an "on/off" switch for many enzymes and signaling molecules. The added phosphate group alters the charge and conformation of the protein, thus affecting its interactions with other molecules.
The Importance of Elemental Balance
The precise ratios of these elements are essential for the proper folding and functioning of proteins. Any deviation from the ideal balance can lead to misfolded proteins, which are often non-functional and may even be harmful to the organism. Such misfolding can be caused by:
-
Genetic mutations: Changes in the DNA sequence can lead to alterations in the amino acid sequence, resulting in altered protein structure and function.
-
Environmental factors: Extreme temperatures, pH changes, and the presence of certain chemicals can disrupt the delicate balance of interactions that maintain protein structure, leading to denaturation (unfolding).
-
Nutritional deficiencies: A lack of essential amino acids can hinder protein synthesis and lead to the production of incomplete or malfunctioning proteins.
Consequences of Elemental Imbalance
The consequences of altered protein structure and function due to elemental imbalance can range from mild to severe, depending on the specific protein affected.
-
Enzyme malfunction: If enzymes are misfolded, they might lose their catalytic activity, disrupting crucial metabolic processes.
-
Structural defects: Structural proteins, like collagen, may lose their integrity, leading to weakened tissues and organs.
-
Disease development: Misfolded proteins are implicated in various diseases, including Alzheimer's disease, Parkinson's disease, and cystic fibrosis.
Conclusion: The Exquisite Interplay of Elements
Proteins are remarkable molecules whose function depends critically on the precise composition and arrangement of their constituent elements. The ‘big four’ – carbon, hydrogen, oxygen, and nitrogen – form the backbone and essential functional groups of amino acids, while sulfur and phosphorus contribute specific functionalities and regulatory roles. Understanding the interplay of these elements is fundamental to understanding the intricate workings of life itself, and disruptions in elemental balance can lead to various debilitating conditions. Further research into protein structure and function continues to illuminate the precise mechanisms by which these elements contribute to the complexity and diversity of life. The more we understand the importance of these elements, the better equipped we are to address issues related to protein misfolding and develop treatments for related diseases. This intricate dance of elements underscores the remarkable elegance and efficiency of biological systems.
Latest Posts
Latest Posts
-
Describe The Sampling Distribution Of P Hat
Mar 25, 2025
-
How To Determine State Of Matter In Chemical Equation
Mar 25, 2025
-
Resistors In Series And Parallel Calculator
Mar 25, 2025
-
Where Are Breathing Control Centers Located
Mar 25, 2025
-
Most Digestion And Absorption Of Food Occurs In The
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Element Is Found In Proteins . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
