How To Determine State Of Matter In Chemical Equation
Muz Play
Mar 25, 2025 · 6 min read
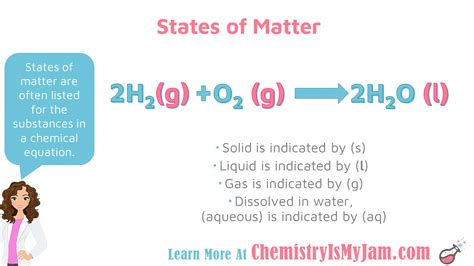
Table of Contents
How to Determine the State of Matter in a Chemical Equation
Determining the state of matter of reactants and products in a chemical equation is crucial for a complete and accurate representation of a chemical reaction. It provides vital information about the reaction conditions, the physical changes involved, and helps predict the outcome. This comprehensive guide delves into various methods and techniques to identify the states of matter, offering a clear understanding for students and enthusiasts alike.
Understanding States of Matter
Before diving into determining states of matter in chemical equations, let's review the fundamental states:
- Solid (s): Solids have a definite shape and volume. Their particles are tightly packed and have strong intermolecular forces.
- Liquid (l): Liquids have a definite volume but take the shape of their container. Particles are closer than in gases but further apart than in solids, with weaker intermolecular forces than solids.
- Gas (g): Gases have neither a definite shape nor volume; they expand to fill their container. Particles are far apart with weak intermolecular forces.
- Aqueous (aq): This state refers to a substance dissolved in water. It's crucial to differentiate this from the liquid state as the properties of the dissolved substance can be significantly different from its pure liquid form.
Methods for Determining States of Matter
Several methods help determine the state of matter of substances involved in a chemical reaction. These include:
1. Observing the Reaction Conditions
The conditions under which a reaction occurs, specifically temperature and pressure, significantly influence the state of matter of reactants and products.
- Temperature: A substance that's solid at room temperature might become liquid or gaseous at higher temperatures. For example, water (H₂O) is liquid at room temperature but becomes a gas (steam) above 100°C at standard pressure.
- Pressure: Pressure affects the state of matter, particularly for gases. Increasing pressure can force a gas to liquefy or even solidify. Conversely, decreasing pressure can cause a liquid to vaporize.
2. Referencing Physical Data
Extensive databases and reference materials provide physical properties of substances, including their melting and boiling points. These points are critical in determining the state of matter at a specific temperature.
- Melting Point: The temperature at which a solid transitions to a liquid.
- Boiling Point: The temperature at which a liquid transitions to a gas.
By comparing the reaction temperature to the melting and boiling points of the substances, you can deduce their state of matter. For example, if the reaction occurs at 25°C and the melting point of a substance is 100°C, it will be solid at reaction conditions.
3. Contextual Clues within the Chemical Equation or Problem
Sometimes, the problem itself or the context of the chemical equation provides clues to the states of matter. Look for phrases like:
- "Dissolved in water": This indicates an aqueous (aq) solution.
- "Solid precipitate forms": This suggests a solid (s) product.
- "Gas is evolved": This indicates a gaseous (g) product.
These hints assist in accurately assigning the states of matter.
4. Understanding Reaction Types
The type of chemical reaction can often provide insights into the states of matter of the reactants and products.
- Precipitation Reactions: These reactions generally involve aqueous solutions, resulting in the formation of a solid precipitate (s).
- Gas-Evolution Reactions: These reactions often produce a gaseous product (g).
- Acid-Base Reactions: These typically involve aqueous solutions and may or may not produce a gas.
- Combustion Reactions: These reactions usually involve a fuel (solid, liquid, or gas) reacting with oxygen gas (g) to produce carbon dioxide gas (g) and water (g or l, depending on the temperature).
Knowing the typical characteristics of these reaction types can assist in predicting the states of matter.
Practical Application: Examples
Let's apply these methods to some chemical equations:
Example 1: Combustion of Methane
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g)
In this equation, we can infer the states of matter based on the common knowledge of these substances under standard conditions. Methane (CH₄), oxygen (O₂), and carbon dioxide (CO₂) are all gases at room temperature. Water (H₂O) is a gas (steam) at the high temperature of a combustion reaction.
Example 2: Precipitation Reaction
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
Here, we see aqueous solutions of silver nitrate (AgNO₃) and sodium chloride (NaCl) reacting to form a solid precipitate of silver chloride (AgCl) and an aqueous solution of sodium nitrate (NaNO₃). The state of matter for each reactant and product is clearly indicated.
Example 3: Reaction with Unknown State
Consider the reaction: 2H₂ + O₂ → 2H₂O
Without additional information, we might assume all substances are in the gaseous state under standard conditions. However, if the reaction occurs at room temperature, the water formed would be liquid. Therefore, the complete equation could be:
2H₂(g) + O₂(g) → 2H₂O(l)
This illustrates the importance of considering reaction conditions.
Advanced Considerations: Beyond the Basic States
While solid, liquid, gas, and aqueous are the most common states, other states exist and might be relevant in certain reactions:
- Plasma: A highly ionized gas, characterized by its high energy state.
- Supercritical Fluid: A state beyond the critical point where a substance exhibits properties of both liquid and gas.
- Colloidal Dispersion: A mixture where one substance is dispersed throughout another, like milk or fog.
These specialized states often require detailed knowledge of the reaction conditions and involved substances to determine.
Importance of Correctly Identifying States of Matter
Accurately identifying the states of matter in chemical equations is vital for several reasons:
- Understanding Reaction Mechanisms: The states of matter provide insight into how reactants interact and how products are formed.
- Predicting Reaction Outcomes: Knowing the states of matter helps predict whether a reaction will proceed as expected or result in unexpected products.
- Stoichiometric Calculations: Calculations involving reaction yields and limiting reactants rely on knowing the number of moles of reactants and products, which is influenced by their states.
- Interpreting Experimental Data: Accurate identification of states of matter is fundamental in interpreting experimental observations and comparing them with theoretical predictions.
Conclusion
Determining the state of matter in chemical equations is a fundamental skill in chemistry. By utilizing various methods and considering reaction conditions, contextual clues, and reference materials, one can accurately represent chemical reactions with the correct states of matter for each participant. This comprehensive understanding greatly enhances the interpretation and comprehension of chemical processes. Remember, the more information you have about the reaction conditions and the substances involved, the more confidently you can determine their states of matter and thereby build a more complete picture of the chemical reaction.
Latest Posts
Latest Posts
-
Which Plane Divides The Body Into Superior And Inferior Sections
Mar 26, 2025
-
Difference Between Substrate Level Phosphorylation And Oxidative Phosphorylation
Mar 26, 2025
-
5 Steps Of The Listening Process
Mar 26, 2025
-
How To Determine If A Transformation Is Linear
Mar 26, 2025
-
How Do You Know If A Reaction Is Redox
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How To Determine State Of Matter In Chemical Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
