Is Chemical Energy Potential Or Kinetic Energy
Muz Play
Mar 23, 2025 · 5 min read
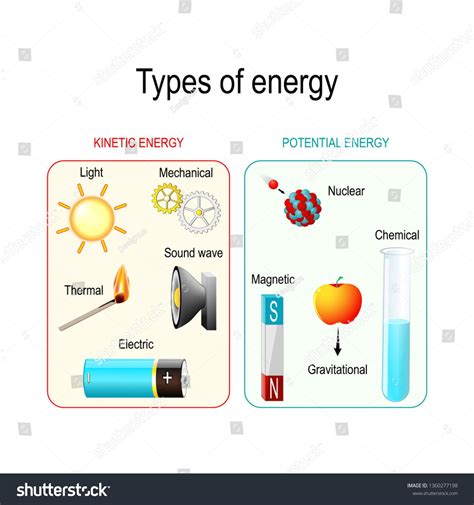
Table of Contents
Is Chemical Energy Potential or Kinetic Energy? Unpacking the Fundamentals
The question of whether chemical energy is potential or kinetic energy is a nuanced one, often leading to confusion. While a simplistic answer might lean towards potential energy, the reality is far more complex and fascinating. Understanding this requires delving into the fundamental concepts of energy, its various forms, and how they interrelate within chemical systems. This article will explore this fascinating topic, providing a comprehensive explanation accessible to both beginners and those with a more advanced scientific background.
Understanding Energy: Potential vs. Kinetic
Before diving into the specifics of chemical energy, let's establish a clear understanding of potential and kinetic energy.
Potential energy is stored energy that has the potential to be converted into other forms of energy, such as kinetic energy. Think of a stretched rubber band or a rock perched on a cliff. These objects possess potential energy due to their position or configuration. In the case of the rubber band, it's the stored elastic potential energy; for the rock, it's gravitational potential energy. This energy is released when the object changes its state – the rubber band snaps back, and the rock falls.
Kinetic energy, on the other hand, is the energy of motion. Any object in motion possesses kinetic energy. The faster the object moves and the greater its mass, the higher its kinetic energy. A speeding car, a flying bird, or even the molecules vibrating within a substance all possess kinetic energy.
The Nature of Chemical Energy
Chemical energy is the energy stored within the bonds of atoms and molecules. These bonds represent a specific arrangement of atoms, and this arrangement dictates the amount of potential energy stored within the chemical system. It's the potential energy associated with the relative positions of atoms and electrons within a molecule. Stronger bonds hold more potential energy, and weaker bonds hold less.
Consider a molecule of methane (CH₄). The carbon atom shares electrons with four hydrogen atoms, forming strong covalent bonds. This arrangement of atoms represents a state of relatively low energy. However, when this methane molecule reacts with oxygen, breaking and forming new bonds, this stored potential energy is released. The released potential energy is converted to kinetic energy during the reaction.
Chemical reactions are the key to understanding the transformation of chemical potential energy. Chemical reactions involve the breaking and forming of chemical bonds. When bonds break, energy is absorbed (endothermic reaction), while when bonds form, energy is released (exothermic reaction). This energy exchange is the essence of chemical energy transformation.
The Role of Electrons and Electron Configuration
The key to understanding chemical potential energy lies in the behavior of electrons. Electrons occupy specific energy levels or orbitals around the nucleus of an atom. The arrangement of electrons in these orbitals determines the stability and reactivity of an atom or molecule.
Atoms strive for the most stable electron configuration, often achieving this by forming chemical bonds with other atoms. When bonds form, electrons are shared or transferred, resulting in a lower overall energy state for the system. This energy difference between the initial and final states is the chemical potential energy that is either released or absorbed during a reaction.
Chemical Energy: Potential Energy Dominated
While the molecules themselves possess kinetic energy due to their movement (vibrational, rotational, and translational), the dominant form of energy inherent within the chemical structure is potential energy. The energy stored in chemical bonds, ready to be released or absorbed during a reaction, is undeniably a form of potential energy. The chemical potential energy is latent, waiting to be transformed.
The Kinetic Component: A Subtle Nuance
Although the primary form of energy associated with chemical substances is potential energy, a secondary kinetic component exists. The atoms and molecules within a substance are constantly in motion, exhibiting vibrational, rotational, and translational kinetic energy. This thermal kinetic energy contributes to the overall energy of the system, but it’s generally smaller in magnitude compared to the potential energy stored within the chemical bonds, especially at normal temperatures.
However, this kinetic energy plays a crucial role in initiating chemical reactions. For a reaction to occur, molecules need to collide with sufficient energy to overcome the activation energy barrier. This activation energy represents the minimum energy required to break existing bonds and initiate the reaction. The higher the temperature, the greater the kinetic energy of the molecules, and the more likely a reaction is to proceed.
Examples illustrating Chemical Potential Energy
Let's consider some everyday examples to solidify our understanding:
-
Burning wood: The wood contains chemical potential energy stored in the cellulose and lignin molecules. When burned, these bonds are broken, and new bonds are formed with oxygen, releasing the stored potential energy as heat and light (kinetic energy).
-
Batteries: Batteries store chemical potential energy in the form of chemical reactants. When the battery is connected to a circuit, a chemical reaction occurs, converting chemical potential energy into electrical energy (a form of kinetic energy), which then powers devices.
-
Food: The food we consume contains chemical potential energy stored in carbohydrates, fats, and proteins. Our bodies metabolize these molecules, breaking the bonds and releasing the stored energy in the form of ATP (adenosine triphosphate), which fuels our bodily functions.
Conclusion: Chemical Energy as Primarily Potential Energy
In conclusion, while chemical systems do possess kinetic energy due to the movement of their constituent molecules, chemical energy is fundamentally a form of potential energy. It's the energy stored within the bonds of atoms and molecules, ready to be released or absorbed during chemical reactions. The transformation of this potential energy into other forms, such as kinetic energy (heat, light, motion), is the driving force behind countless natural phenomena and technological applications.
Understanding the distinction between potential and kinetic energy, and how they interplay within chemical systems, is crucial for comprehending the world around us. From the burning of fuels to the processes within living organisms, chemical energy fuels a multitude of processes essential to life and technology. This subtle yet fundamental understanding unlocks deeper insights into the marvels of chemistry and the dynamics of our universe.
Latest Posts
Latest Posts
-
2 Sample Z Test For Proportions
Mar 25, 2025
-
Last Of Five Rhyming Greek Letters
Mar 25, 2025
-
Rusting Of Iron Is Chemical Or Physical Change
Mar 25, 2025
-
Differential Equation For Newtons Law Of Cooling
Mar 25, 2025
-
In Aerobic Cellular Respiration What Are The 3 Major Steps
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Is Chemical Energy Potential Or Kinetic Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
