Rusting Of Iron Is Chemical Or Physical Change
Muz Play
Mar 25, 2025 · 6 min read
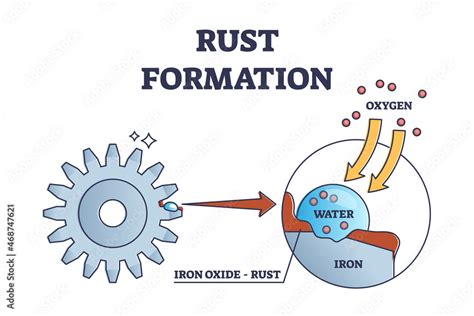
Table of Contents
Rusting of Iron: A Chemical Change Explained
The question of whether rusting is a chemical or physical change is a common one, especially in introductory science classes. The answer, unequivocally, is that rusting is a chemical change. This seemingly simple statement belies a complex process involving several intricate chemical reactions. This article will delve deep into the science behind rust formation, explaining why it's a chemical change and exploring the factors that influence its rate and severity. We'll also explore the differences between chemical and physical changes to solidify our understanding.
Understanding Chemical vs. Physical Changes
Before diving into the specifics of rust formation, it's crucial to establish a clear understanding of the difference between chemical and physical changes. A physical change alters the form or appearance of a substance but doesn't change its chemical composition. Think of cutting a piece of paper, melting ice, or dissolving sugar in water. The paper, ice, and sugar remain chemically the same; only their physical state or arrangement has changed.
A chemical change, on the other hand, involves a transformation of the substance's chemical composition. New substances with different properties are formed. Examples include burning wood, cooking an egg, or digesting food. In each case, the starting materials are transformed into entirely new substances.
The Chemistry of Rust Formation (Oxidation)
Rust, chemically known as iron(III) oxide (Fe₂O₃), is the product of a chemical reaction between iron (Fe) and oxygen (O₂) in the presence of water (H₂O). This reaction is a type of oxidation, a process where a substance loses electrons. In rusting, iron atoms lose electrons to oxygen atoms, forming iron ions and oxide ions. The process isn't instantaneous; it's a complex electrochemical reaction involving several steps:
1. Oxidation of Iron:
The iron atoms on the surface of the metal lose electrons and become positively charged iron(II) ions (Fe²⁺):
Fe(s) → Fe²⁺(aq) + 2e⁻
This process occurs more readily in acidic environments, where the presence of H⁺ ions facilitates electron transfer. This is why acidic rain accelerates rust formation.
2. Reduction of Oxygen:
Simultaneously, oxygen molecules in the air gain electrons and react with water to form hydroxide ions (OH⁻):
O₂(g) + 2H₂O(l) + 4e⁻ → 4OH⁻(aq)
This reaction is a reduction process, as oxygen gains electrons. The electrons released during iron's oxidation are consumed in this reduction process.
3. Formation of Iron(III) Oxide:
The iron(II) ions (Fe²⁺) formed in the first step further react with hydroxide ions (OH⁻) and oxygen to form iron(III) oxide (Fe₂O₃), which is rust:
4Fe²⁺(aq) + 4OH⁻(aq) + O₂(g) + 2H₂O(l) → 2Fe₂O₃·xH₂O(s)
The ‘·xH₂O’ indicates that rust is hydrated, meaning it incorporates water molecules into its structure. The exact amount of water incorporated can vary, leading to different forms of rust.
Factors Affecting Rusting: An Exploration of Rate and Severity
The rate at which iron rusts is influenced by several factors:
1. Presence of Water and Oxygen:
Water and oxygen are essential for rust formation. The higher the humidity and the greater the exposure to oxygen, the faster the rusting process. Completely dry iron, or iron in a vacuum, will not rust.
2. Acidity:
As mentioned earlier, acidic conditions accelerate rust formation. The presence of acids, like those found in acid rain or in saltwater, lowers the pH and increases the rate of electron transfer.
3. Temperature:
Higher temperatures generally increase the rate of chemical reactions, including rusting. The increased kinetic energy of molecules at higher temperatures leads to more frequent and energetic collisions, accelerating the reaction.
4. Presence of Electrolytes:
Electrolytes, which are substances that conduct electricity when dissolved in water, accelerate rusting. Saltwater, for example, is a strong electrolyte and significantly accelerates rust formation. This is why coastal areas experience rapid corrosion of iron structures.
5. Surface Area:
A larger surface area of iron exposed to the environment leads to faster rusting. A finely divided powder of iron will rust much more quickly than a solid block of iron of the same mass.
Preventing Rust: Protective Measures
Given the destructive nature of rust, understanding its formation is crucial for implementing effective preventative measures. Several strategies can significantly reduce or prevent rust:
1. Coatings:
Applying protective coatings, such as paint, varnish, or oil, creates a barrier between the iron and the environment, preventing exposure to oxygen and water.
2. Galvanization:
Galvanization involves coating iron with a layer of zinc. Zinc is more reactive than iron, meaning it will oxidize preferentially, protecting the iron underneath. This sacrificial protection prevents rust.
3. Cathodic Protection:
This method uses a more reactive metal, like magnesium or zinc, as a sacrificial anode to protect the iron. The more reactive metal corrodes instead of the iron.
4. Alloying:
Adding other elements to iron, such as chromium (forming stainless steel), increases its resistance to corrosion. The chromium forms a protective oxide layer that prevents further oxidation.
Rusting as an Electrochemical Process: A Deeper Dive
The rusting process is fundamentally an electrochemical process. This means it involves the flow of electrons between different parts of the iron surface. Microscopic variations in the iron's surface create anodic and cathodic regions.
- Anode: This is where oxidation occurs, and iron loses electrons. Fe²⁺ ions are released into the solution.
- Cathode: This is where reduction occurs, and oxygen gains electrons. Hydroxide ions (OH⁻) are formed.
The electrons flow from the anode to the cathode through the iron itself, creating an electrical current. The presence of an electrolyte (like water with dissolved salts) facilitates this electron flow, completing the circuit and accelerating the rusting process. This electrochemical nature explains why rust doesn't form uniformly across the surface but often appears in localized areas.
Real-World Examples and Implications
The implications of iron rusting are widespread and significant:
- Infrastructure damage: Rusting of bridges, buildings, and pipelines leads to structural weakening and potential failures, resulting in costly repairs and safety hazards.
- Vehicle damage: Rust significantly damages automobiles, reducing their lifespan and requiring expensive repairs or replacements.
- Economic losses: The cost of rust prevention and repair is substantial globally, impacting various industries.
- Environmental impact: The production and disposal of iron and its rust-related byproducts have environmental consequences.
Conclusion: Rusting - An Irrefutable Chemical Change
In conclusion, the rusting of iron is unequivocally a chemical change. It involves a complex series of redox reactions resulting in the formation of a new substance, iron(III) oxide (Fe₂O₃), with significantly different properties than the original iron. Understanding this chemical process, along with the factors influencing its rate, is crucial for developing effective strategies to prevent rust and mitigate its destructive effects. The widespread implications of rust highlight the importance of ongoing research and innovation in corrosion prevention technologies. From protecting our infrastructure to extending the lifespan of valuable assets, preventing rust remains a significant challenge and an ongoing area of scientific and engineering focus.
Latest Posts
Latest Posts
-
Which Correctly Summarizes The Trend In Electron Affinity
Mar 25, 2025
-
Delta H Delta S Delta G Chart
Mar 25, 2025
-
A Chemical Equation Is Balanced When
Mar 25, 2025
-
What Is The Average Kinetic Energy
Mar 25, 2025
-
What Are The Building Blocks Of Macromolecules
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Rusting Of Iron Is Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
