Delta H Delta S Delta G Chart
Muz Play
Mar 25, 2025 · 6 min read
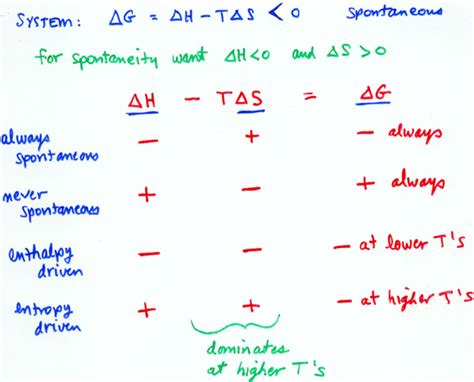
Table of Contents
Understanding Gibbs Free Energy: A Deep Dive into ΔH, ΔS, and ΔG
Thermodynamics, the study of energy and its transformations, is fundamental to understanding chemical and physical processes. A critical concept within thermodynamics is Gibbs Free Energy (G), which dictates the spontaneity of a reaction or process. Gibbs Free Energy is intimately linked to enthalpy (H), entropy (S), and temperature (T) through the equation: ΔG = ΔH - TΔS. This article will explore the relationships between ΔH, ΔS, and ΔG, providing a comprehensive understanding of their interplay and offering a visual representation through a conceptual chart. We will also delve into various applications and examples.
Understanding the Key Terms: Enthalpy (ΔH), Entropy (ΔS), and Gibbs Free Energy (ΔG)
Before diving into the intricacies of their relationship, let's define each term individually:
Enthalpy (ΔH): A Measure of Heat Content
Enthalpy (ΔH) represents the heat content of a system at constant pressure. A negative ΔH indicates an exothermic reaction, where heat is released to the surroundings (e.g., combustion). A positive ΔH indicates an endothermic reaction, where heat is absorbed from the surroundings (e.g., melting ice). Enthalpy changes are crucial for understanding the energy balance of a reaction.
Entropy (ΔS): A Measure of Disorder
Entropy (ΔS) measures the degree of randomness or disorder within a system. A positive ΔS indicates an increase in disorder, meaning the system becomes more random (e.g., melting a solid into a liquid). A negative ΔS indicates a decrease in disorder, meaning the system becomes more ordered (e.g., freezing a liquid into a solid). Entropy is a key factor determining the spontaneity of a process. The Second Law of Thermodynamics states that the total entropy of an isolated system can only increase over time.
Gibbs Free Energy (ΔG): Predicting Spontaneity
Gibbs Free Energy (ΔG) combines enthalpy and entropy to predict the spontaneity of a process at constant temperature and pressure. It represents the maximum amount of energy available from a system to do useful work. The sign of ΔG determines spontaneity:
- ΔG < 0 (negative): The process is spontaneous (occurs naturally without external intervention).
- ΔG > 0 (positive): The process is non-spontaneous (requires external energy input to occur).
- ΔG = 0 (zero): The process is at equilibrium; the forward and reverse reactions occur at equal rates.
The Interplay of ΔH, ΔS, and ΔG: A Conceptual Chart
The relationship between ΔH, ΔS, and ΔG can be visualized using a chart that categorizes reactions based on the signs of ΔH and ΔS. This chart helps predict the spontaneity of a reaction at different temperatures:
| ΔS > 0 (Increase in Disorder) | ΔS < 0 (Decrease in Disorder) | |
|---|---|---|
| ΔH < 0 (Exothermic) | ΔG < 0 (Always Spontaneous) | ΔG < 0 at high T, ΔG > 0 at low T |
| ΔH > 0 (Endothermic) | ΔG < 0 at high T, ΔG > 0 at low T | ΔG > 0 (Always Non-Spontaneous) |
Let's analyze each quadrant:
- Quadrant 1 (ΔH < 0, ΔS > 0): Always Spontaneous
Reactions in this quadrant are both exothermic (release heat) and increase disorder. These reactions are always spontaneous at all temperatures because both enthalpy and entropy contribute favorably to a negative ΔG. Examples include combustion reactions and many dissolution processes.
- Quadrant 2 (ΔH < 0, ΔS < 0): Spontaneous at High Temperatures
These reactions are exothermic but decrease disorder. At high temperatures, the -TΔS term becomes large enough to overcome the negative ΔH, resulting in a negative ΔG and spontaneity. At low temperatures, the -TΔS term is small, making ΔG positive and the reaction non-spontaneous. A classic example is the condensation of a gas to a liquid.
- Quadrant 3 (ΔH > 0, ΔS > 0): Spontaneous at High Temperatures
These reactions are endothermic and increase disorder. At high temperatures, the positive entropy term outweighs the positive enthalpy term, resulting in a negative ΔG and spontaneity. At low temperatures, the -TΔS term is too small to overcome the positive ΔH, rendering the reaction non-spontaneous. A common example is the melting of ice.
- Quadrant 4 (ΔH > 0, ΔS < 0): Never Spontaneous
Reactions in this quadrant are both endothermic and decrease disorder. Both enthalpy and entropy contribute unfavorably to ΔG, making the reaction always non-spontaneous at all temperatures. Examples include the decomposition of many compounds under standard conditions.
Applications and Examples of ΔH, ΔS, and ΔG
Understanding the relationship between ΔH, ΔS, and ΔG has numerous applications across various fields:
1. Predicting Reaction Spontaneity:
The most direct application is predicting whether a reaction will proceed spontaneously under given conditions. By calculating ΔH and ΔS, one can determine the spontaneity of a reaction at various temperatures using the Gibbs Free Energy equation.
Example: Consider the dissolution of ammonium nitrate in water. This process is endothermic (ΔH > 0) and increases disorder (ΔS > 0). At room temperature, the reaction is spontaneous (ΔG < 0) because the increase in entropy outweighs the endothermic nature of the process.
2. Phase Transitions:
The relationship between ΔH, ΔS, and ΔG is crucial for understanding phase transitions (e.g., melting, boiling, sublimation). The temperature at which a phase transition occurs (e.g., melting point) is the temperature where ΔG = 0.
3. Chemical Equilibrium:
At equilibrium, ΔG = 0. The equilibrium constant (K) is related to the standard Gibbs free energy change (ΔG°) through the equation: ΔG° = -RTlnK, where R is the gas constant and T is the temperature.
4. Electrochemistry:
In electrochemistry, ΔG is related to the cell potential (E) through the equation: ΔG = -nFE, where n is the number of electrons transferred and F is the Faraday constant. This allows for the calculation of cell potential from thermodynamic data.
5. Biochemical Processes:
Understanding Gibbs Free Energy is essential in biochemistry, where the spontaneity of metabolic reactions is crucial. Many biochemical reactions are coupled with ATP hydrolysis (a highly exergonic reaction, ΔG << 0) to drive otherwise non-spontaneous processes.
Limitations and Considerations
While the ΔG = ΔH - TΔS equation provides a powerful framework for predicting spontaneity, it has limitations:
- It only predicts spontaneity under constant temperature and pressure. Real-world reactions often occur under non-constant conditions.
- It doesn't provide information about the reaction rate. A spontaneous reaction might proceed extremely slowly.
- It assumes ideal behavior. Deviations from ideal behavior can affect the accuracy of the predictions.
Conclusion: A Powerful Tool for Understanding Chemical Processes
The Gibbs Free Energy equation, incorporating enthalpy, entropy, and temperature, provides a powerful tool for understanding the spontaneity of chemical and physical processes. By analyzing the signs of ΔH and ΔS and using the conceptual chart provided, one can predict the spontaneity of a reaction under various conditions. While the equation has limitations, it remains a cornerstone of thermodynamics and has broad applications across various scientific disciplines. Understanding this fundamental relationship is essential for anyone seeking a deeper understanding of the natural world.
Latest Posts
Latest Posts
-
How Do You Calculate The Heat Capacity Of A Calorimeter
Mar 26, 2025
-
Mendels Dihybrid Crosses Supported The Independent Hypothesis
Mar 26, 2025
-
Is Kinetic Energy Conserved In An Elastic Collision
Mar 26, 2025
-
Surplus And Shortage On A Graph
Mar 26, 2025
-
Indicate Whether Or Not The Following Molecules Are Chiral
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Delta H Delta S Delta G Chart . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
