Indicate Whether Or Not The Following Molecules Are Chiral.
Muz Play
Mar 26, 2025 · 5 min read
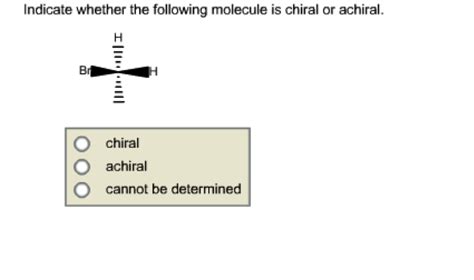
Table of Contents
Indicating Chirality in Molecules: A Comprehensive Guide
Chirality, a fundamental concept in organic chemistry, refers to the property of a molecule that is not superimposable on its mirror image. Think of your hands – they are mirror images of each other, but you can't perfectly overlay one onto the other. This same principle applies to chiral molecules. Understanding chirality is crucial in various fields, including pharmacology, biochemistry, and materials science, as chiral molecules often exhibit different properties and interactions. This article will delve into the intricacies of chirality, providing a comprehensive guide to determine whether various molecules are chiral or achiral.
What Makes a Molecule Chiral?
The presence of a stereocenter or chiral center is the primary indicator of chirality. A stereocenter is typically a carbon atom bonded to four different substituents. This tetrahedral arrangement creates two non-superimposable mirror images, known as enantiomers. However, chirality extends beyond just carbon atoms. Other atoms like phosphorus, sulfur, and nitrogen can also act as stereocenters under specific conditions.
Key Factors Determining Chirality:
- Asymmetric Carbon: The most common type of stereocenter is a carbon atom bonded to four different groups. This asymmetry is the key to chirality.
- Presence of a Plane of Symmetry: If a molecule possesses a plane of symmetry (a plane that divides the molecule into two mirror-image halves), it is achiral (not chiral). The presence of a plane of symmetry renders the molecule superimposable on its mirror image.
- Conformational Isomers: Conformational isomers (rotamers) are different spatial arrangements of a molecule that can be interconverted by rotation around single bonds. These are generally not considered chiral, as they are readily interconvertible.
- Stereocenters and Chirality: The number of stereocenters does not directly correlate with the number of stereoisomers. A molecule with 'n' stereocenters can have a maximum of 2<sup>n</sup> stereoisomers. However, the presence of symmetry elements can reduce this number.
Identifying Chiral Molecules: Practical Examples
Let's analyze several molecules to illustrate the principles of chirality identification.
Example 1: 2-Bromobutane
The central carbon atom in 2-bromobutane is bonded to four different groups: a bromine atom, a methyl group (–CH3), an ethyl group (–CH2CH3), and a hydrogen atom. This carbon atom is a stereocenter. Therefore, 2-bromobutane is chiral. It exists as a pair of enantiomers.
Example 2: Chloromethane
Chloromethane (CH3Cl) has a central carbon atom bonded to three hydrogen atoms and one chlorine atom. All four groups are not different, making it an achiral molecule. Therefore, chloromethane is achiral.
Example 3: 1,2-Dibromopropane
1,2-Dibromopropane possesses two chiral centers (two carbons attached to four different groups). However, the molecule has a plane of symmetry; therefore it is meso, a special type of achiral compound with chiral centers. 1,2-Dibromopropane is achiral despite having two stereocenters.
Example 4: Tartaric Acid
Tartaric acid has two chiral centers, but it exhibits different stereoisomers. The molecule exists in three forms: two enantiomers (D-tartaric acid and L-tartaric acid) and a meso compound (meso-tartaric acid). The meso form is achiral due to its internal plane of symmetry.
Example 5: 1-Bromo-1-chloropropane
In 1-bromo-1-chloropropane, the central carbon atom is bonded to a bromine atom, a chlorine atom, a methyl group, and a hydrogen atom. All four substituents are different; thus, it possesses a stereocenter. Therefore, 1-bromo-1-chloropropane is chiral.
Example 6: 1,1-Dibromopropane
The central carbon atom in 1,1-dibromopropane is bonded to two identical bromine atoms, a methyl group, and a hydrogen atom. Because it has two identical bromine atoms, it does not have a stereocenter. Therefore, 1,1-dibromopropane is achiral.
Advanced Concepts in Chirality
Beyond the basic principles, there are more nuanced aspects of chirality to consider:
Allenes and Other Non-Tetrahedral Stereocenters
Chirality is not limited to tetrahedral carbon atoms. Allenes, which contain two adjacent double bonds (C=C=C), can exhibit chirality even without having a tetrahedral stereocenter. The arrangement of substituents around the allene creates a chiral axis. Other molecules with chiral axes or planar chirality are possible.
Diastereomers
When a molecule has more than one stereocenter, it can exist as different stereoisomers that are not mirror images. These are called diastereomers. Diastereomers have different physical and chemical properties, unlike enantiomers which have identical properties except for their interaction with plane-polarized light.
Racemic Mixtures
A racemic mixture is a 50:50 mixture of two enantiomers. It is optically inactive because the rotations of the enantiomers cancel each other out.
Resolution of Enantiomers
Separating a racemic mixture into its individual enantiomers is called resolution. This is often achieved through various techniques such as chiral chromatography or the use of resolving agents.
Applications of Chirality
Chirality plays a significant role in various fields:
- Pharmacology: Many drugs are chiral, and the different enantiomers can exhibit vastly different pharmacological effects. One enantiomer might be therapeutically active, while the other could be inactive or even toxic.
- Biochemistry: Biological molecules are often chiral, and their interactions are highly stereospecific. Enzymes, for example, often only interact with one enantiomer of a substrate.
- Materials Science: Chiral molecules can be used to create materials with unique optical properties or to control the crystallization of other molecules.
Conclusion
Determining whether a molecule is chiral or achiral involves a systematic assessment of its structure. Identifying stereocenters, checking for planes of symmetry, and understanding advanced concepts like diastereomers and racemic mixtures are crucial. The implications of chirality extend far beyond the realm of organic chemistry, impacting fields like pharmacology, biochemistry, and materials science. By understanding and applying the principles discussed in this article, you can confidently assess the chirality of molecules and appreciate the significant role this concept plays in the natural world and beyond.
Latest Posts
Latest Posts
-
How To Find Point Of Tangency
Mar 29, 2025
-
How Do You Calculate Potential Difference
Mar 29, 2025
-
How To Round To Four Decimal Places
Mar 29, 2025
-
Reaction Of Benzoic Acid And Naoh
Mar 29, 2025
-
What Type Of Compounds Dissolve To Become Electrolyte
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Indicate Whether Or Not The Following Molecules Are Chiral. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
