Reaction Of Benzoic Acid And Naoh
Muz Play
Mar 29, 2025 · 5 min read
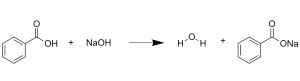
Table of Contents
The Reaction of Benzoic Acid and NaOH: A Deep Dive into Neutralization and Salt Formation
Benzoic acid, a simple aromatic carboxylic acid, readily reacts with sodium hydroxide (NaOH), a strong base, in a classic acid-base neutralization reaction. This reaction is fundamental in organic chemistry and has widespread applications in various fields, from synthesis to analysis. Understanding the reaction mechanism, product properties, and practical implications is crucial for anyone working with these chemicals. This article delves into the intricacies of this reaction, providing a comprehensive overview suitable for both students and professionals.
The Acid-Base Neutralization Reaction: A Detailed Look
The reaction between benzoic acid (C₇H₆O₂) and sodium hydroxide (NaOH) is a straightforward acid-base neutralization. Benzoic acid, possessing a carboxylic acid (-COOH) functional group, acts as a Brønsted-Lowry acid, readily donating a proton (H⁺). Sodium hydroxide, a strong base, readily accepts this proton, acting as a Brønsted-Lowry base. The reaction proceeds as follows:
C₇H₆O₂ (aq) + NaOH (aq) → C₇H₅O₂⁻Na⁺ (aq) + H₂O (l)
This equation represents the overall reaction. Let's break down the mechanism in more detail:
Step-by-Step Mechanism
-
Proton Transfer: The hydroxide ion (OH⁻) from NaOH attacks the acidic proton on the carboxylic acid group of benzoic acid. This proton transfer is the key step in the neutralization reaction. The oxygen atom of the hydroxide ion forms a bond with the hydrogen atom, while the O-H bond in the carboxylic acid group breaks.
-
Formation of Benzoate Ion: The proton transfer leads to the formation of a benzoate ion (C₇H₅O₂⁻), the conjugate base of benzoic acid. This ion carries a negative charge, localized on the carboxylate group (-COO⁻).
-
Formation of Sodium Benzoate: The sodium cation (Na⁺), the counterion from NaOH, electrostatically interacts with the negatively charged benzoate ion. This interaction forms an ionic bond, resulting in the formation of sodium benzoate (C₇H₅O₂⁻Na⁺), a salt.
-
Water Formation: The remaining hydrogen ion (H⁺) from benzoic acid combines with the hydroxide ion (OH⁻) from NaOH to form a molecule of water (H₂O). This is the neutralization aspect of the reaction.
Understanding the Equilibrium
While the reaction proceeds largely to completion due to the strong basicity of NaOH, it's important to remember that it's an equilibrium reaction. The equilibrium constant (K) for this reaction is very large, indicating that the products (sodium benzoate and water) are strongly favored.
Properties of the Reactants and Products
Understanding the properties of the reactants and products is crucial for understanding the reaction and its applications.
Benzoic Acid:
- Appearance: White crystalline powder.
- Solubility: Slightly soluble in water, more soluble in organic solvents.
- Acidity: Weak acid (pKa ≈ 4.2). This means it doesn't fully dissociate in water, only partially releasing H⁺ ions.
- Applications: Preservative in food and beverages, intermediate in the synthesis of other compounds.
Sodium Hydroxide:
- Appearance: White solid, highly deliquescent (absorbs moisture from the air).
- Solubility: Highly soluble in water.
- Basicity: Strong base, completely dissociates in water to release OH⁻ ions.
- Applications: Widely used in various industries, including chemical processing, soap manufacturing, and drain cleaning.
Sodium Benzoate:
- Appearance: White crystalline powder.
- Solubility: Highly soluble in water.
- Properties: Ionic compound; stable under normal conditions.
- Applications: Food preservative, pharmaceutical intermediate. It's far more soluble in water compared to benzoic acid, making it more effective as a preservative in aqueous solutions.
Practical Applications and Significance
The reaction between benzoic acid and NaOH has several important applications:
1. Synthesis of Sodium Benzoate:
The primary application is the large-scale synthesis of sodium benzoate, a widely used food preservative. Its high solubility in water makes it ideal for preserving aqueous food products. The reaction provides a straightforward and efficient method for producing high-purity sodium benzoate.
2. Pharmaceutical Industry:
Sodium benzoate is used as an intermediate in the synthesis of various pharmaceuticals. Its properties allow for easy incorporation into various formulations.
3. Acid-Base Titrations:
This reaction is used in quantitative analysis, specifically acid-base titrations. By carefully measuring the amount of NaOH needed to neutralize a known amount of benzoic acid, one can determine the concentration of the acid. This is a common technique in analytical chemistry.
4. Understanding Acid-Base Chemistry:
The reaction serves as an excellent example for illustrating the principles of acid-base chemistry, including neutralization, equilibrium, and the behavior of weak acids and strong bases.
Experimental Considerations and Safety Precautions
Performing this reaction requires careful attention to safety procedures:
- Protective Gear: Always wear safety goggles, gloves, and a lab coat when handling chemicals.
- Ventilation: Perform the reaction in a well-ventilated area or under a fume hood to avoid inhalation of any vapors.
- Handling NaOH: NaOH is corrosive; handle it with care and avoid skin contact. If contact occurs, immediately flush the affected area with plenty of water.
- Disposal: Dispose of waste materials according to appropriate guidelines. Never pour chemicals down the drain without prior neutralization and proper disposal procedures.
Further Exploration and Related Topics
The reaction of benzoic acid and NaOH opens doors to explore various related topics, including:
- Other Carboxylic Acids: The reaction is applicable to other carboxylic acids, demonstrating the general nature of acid-base neutralization reactions.
- Esterification: Benzoate esters are synthesized using various alcohols. Understanding this reaction provides a broader understanding of carboxylic acid derivatives.
- pH Calculations: Calculating the pH of solutions containing benzoic acid and sodium benzoate requires understanding buffer solutions.
- Spectroscopic Analysis: Techniques like NMR and IR spectroscopy can be used to characterize benzoic acid, NaOH, and sodium benzoate, providing insights into their structure and properties.
Conclusion
The reaction of benzoic acid and NaOH is a fundamental reaction with far-reaching applications. Its simplicity belies the depth of chemical principles involved. Understanding this reaction provides a solid foundation for understanding acid-base chemistry, reaction mechanisms, and the properties of important chemical compounds. Whether you are a student, researcher, or professional in a related field, a thorough understanding of this reaction is invaluable. Always prioritize safety when handling these chemicals and follow appropriate laboratory procedures. By carefully observing the reaction and analyzing the properties of the reactants and products, you gain a comprehensive understanding of chemical reactions and their implications. Remember to always consult reliable resources and adhere to safety protocols when experimenting with chemicals.
Latest Posts
Latest Posts
-
What Are The Differences Between The Pulmonary And Systemic Circulation
Mar 31, 2025
-
A Perfectly Elastic Supply Curve Is
Mar 31, 2025
-
Do Acids And Bases Conduct Electricity
Mar 31, 2025
-
Inverse Relations And Functions Quick Check
Mar 31, 2025
-
Table Salt Is A Pure Substance
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Reaction Of Benzoic Acid And Naoh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
