Do Acids And Bases Conduct Electricity
Muz Play
Mar 31, 2025 · 6 min read
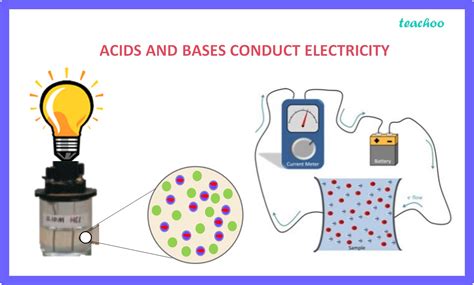
Table of Contents
Do Acids and Bases Conduct Electricity? Exploring the Role of Ions
Acids and bases are fundamental concepts in chemistry, playing crucial roles in numerous natural and industrial processes. One intriguing characteristic shared by many acids and bases is their ability to conduct electricity. This property isn't inherent to all acids and bases, however, and understanding why requires delving into the nature of ionic solutions and the mechanisms of electrical conductivity. This article will explore the conductivity of acids and bases, examining the underlying principles and providing examples to illuminate the concepts.
Understanding Electrical Conductivity
Before diving into the specifics of acids and bases, let's establish a clear understanding of electrical conductivity itself. Electrical conductivity is the ability of a material to conduct electric current. This ability is directly related to the presence and mobility of charged particles, known as ions, within the material. Materials with a high concentration of mobile ions are good conductors of electricity, while those with few or immobile ions are poor conductors, or insulators.
The Role of Ions
Ions are atoms or molecules that carry a net electric charge. Cations are positively charged ions, formed when an atom loses electrons. Anions are negatively charged ions, formed when an atom gains electrons. The movement of these ions under the influence of an electric field constitutes an electric current.
Metals are excellent conductors because they have a "sea" of freely moving electrons. However, for solutions (like acid and base solutions), the conductivity is primarily due to the presence and mobility of ions dissolved in the solvent, typically water.
Conductivity of Acids
Acids, by definition, are substances that donate protons (H⁺ ions) in aqueous solution. The strength of an acid determines the extent of proton donation. Strong acids, like hydrochloric acid (HCl) and sulfuric acid (H₂SO₄), completely dissociate into their ions in water, producing a high concentration of mobile H⁺ and corresponding anions. This high concentration of mobile ions makes strong acid solutions excellent conductors of electricity.
Strong Acid Examples and Conductivity:
- Hydrochloric acid (HCl): HCl → H⁺ + Cl⁻. The complete dissociation of HCl in water results in a significant number of H⁺ and Cl⁻ ions, leading to high conductivity.
- Sulfuric acid (H₂SO₄): H₂SO₄ → 2H⁺ + SO₄²⁻. Similarly, sulfuric acid completely dissociates, producing even more ions per molecule than HCl, resulting in very high conductivity.
- Nitric acid (HNO₃): HNO₃ → H⁺ + NO₃⁻. Another strong acid exhibiting high conductivity due to complete dissociation.
Weak Acid Conductivity:
Weak acids, like acetic acid (CH₃COOH) and carbonic acid (H₂CO₃), only partially dissociate in water. This means that only a small fraction of the acid molecules donate protons, resulting in a lower concentration of ions compared to strong acids. Consequently, weak acid solutions are weaker conductors of electricity than strong acid solutions. The degree of dissociation is often represented by the acid dissociation constant (Ka). A higher Ka value indicates a stronger acid and greater conductivity.
Weak Acid Examples and Conductivity:
- Acetic acid (CH₃COOH): CH₃COOH ⇌ H⁺ + CH₃COO⁻. The equilibrium lies far to the left, meaning only a small percentage of acetic acid molecules dissociate, resulting in low conductivity.
- Carbonic acid (H₂CO₃): H₂CO₃ ⇌ H⁺ + HCO₃⁻. Similar to acetic acid, carbonic acid is a weak acid with limited dissociation and low conductivity.
- Hydrofluoric acid (HF): HF ⇌ H⁺ + F⁻. While HF is a weak acid, it’s a relatively stronger weak acid than others, having a higher conductivity than acetic acid, for instance, but much lower than a strong acid.
Conductivity of Bases
Bases, in contrast to acids, are substances that accept protons (H⁺ ions) or donate hydroxide ions (OH⁻) in aqueous solution. Similar to acids, the strength of a base determines the extent of ionization, influencing its electrical conductivity.
Strong Base Conductivity:
Strong bases, such as sodium hydroxide (NaOH) and potassium hydroxide (KOH), completely dissociate in water, producing a high concentration of OH⁻ ions and corresponding cations (Na⁺ or K⁺). These ions contribute significantly to the electrical conductivity of the solution.
Strong Base Examples and Conductivity:
- Sodium hydroxide (NaOH): NaOH → Na⁺ + OH⁻. The complete dissociation results in high conductivity.
- Potassium hydroxide (KOH): KOH → K⁺ + OH⁻. Similar to NaOH, KOH's complete dissociation leads to high conductivity.
- Calcium hydroxide (Ca(OH)₂): Ca(OH)₂ → Ca²⁺ + 2OH⁻. This strong base produces even more ions per molecule, leading to higher conductivity than NaOH or KOH.
Weak Base Conductivity:
Weak bases, like ammonia (NH₃) and many organic amines, only partially ionize in water. They either accept protons to a limited extent or release hydroxide ions to a smaller degree than strong bases. This results in a lower concentration of ions and thus, lower electrical conductivity compared to strong bases. The base dissociation constant (Kb) is used to quantify the extent of ionization of a weak base.
Weak Base Examples and Conductivity:
- Ammonia (NH₃): NH₃ + H₂O ⇌ NH₄⁺ + OH⁻. Ammonia only partially reacts with water to produce hydroxide ions, leading to low conductivity.
- Methylamine (CH₃NH₂): CH₃NH₂ + H₂O ⇌ CH₃NH₃⁺ + OH⁻. Similar to ammonia, methylamine is a weak base with limited ionization and low conductivity.
- Pyridine (C₅H₅N): C₅H₅N + H₂O ⇌ C₅H₅NH⁺ + OH⁻. Another example of a weak base with low conductivity due to partial ionization.
Factors Affecting Conductivity
Several factors can influence the electrical conductivity of acid and base solutions, including:
- Concentration: Higher concentrations of both acids and bases lead to higher conductivity due to the increased number of ions present.
- Temperature: Increased temperature generally enhances conductivity. Higher temperatures increase the kinetic energy of ions, improving their mobility and thus the current flow.
- Solvent: The nature of the solvent affects the dissociation of acids and bases and hence their conductivity. Water is a common solvent, but other solvents can affect the ion mobility and solubility.
- Strength of Acid/Base: Strong acids and bases conduct electricity better than weak acids and bases due to the greater degree of ionization.
- Presence of other ions: The presence of other dissolved ions in the solution (from salts, for instance) can affect conductivity by increasing the overall ionic strength.
Applications of Conductivity Measurement
The ability of acids and bases to conduct electricity has many practical applications:
- Titrations: Conductivity measurements are used in titrations to determine the equivalence point of acid-base reactions. The change in conductivity during the titration reflects the change in the concentration of ions.
- Water Purity Testing: Conductivity measurements are used to assess the purity of water. Pure water has very low conductivity, while the presence of impurities (like dissolved salts or acids) increases conductivity.
- Soil Testing: Conductivity measurements are employed in agriculture to determine the salinity of the soil, affecting the growth of plants.
- Industrial Processes: Conductivity measurements monitor various industrial processes involving acids and bases, such as chemical synthesis and wastewater treatment.
Conclusion
The electrical conductivity of acids and bases is directly linked to the concentration and mobility of ions in solution. Strong acids and bases, completely dissociating into ions, are excellent conductors. In contrast, weak acids and bases, with limited ionization, exhibit lower conductivity. Various factors, including concentration, temperature, and the nature of the acid or base itself, influence the overall conductivity. Understanding this relationship is crucial in various applications, from chemical analysis to environmental monitoring and industrial processes. By appreciating the fundamental principles connecting ion mobility, ionization, and electrical conductivity, we gain a deeper understanding of the behavior of these essential chemical compounds.
Latest Posts
Latest Posts
-
How Do You Find The Boiling Point Of A Solution
Apr 02, 2025
-
What Happens To Ionization Energy Down A Group
Apr 02, 2025
-
How Many Grams In A Molecule
Apr 02, 2025
-
Is Cl A Metal Or Nonmetal
Apr 02, 2025
-
Microscopic Anatomy And Organization Of Skeletal Muscle
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Do Acids And Bases Conduct Electricity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
