What Type Of Compounds Dissolve To Become Electrolyte
Muz Play
Mar 29, 2025 · 6 min read
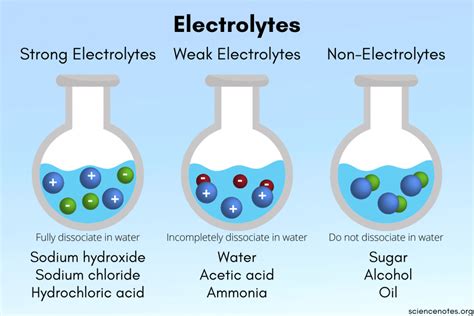
Table of Contents
- What Type Of Compounds Dissolve To Become Electrolyte
- Table of Contents
- What Types of Compounds Dissolve to Become Electrolytes?
- The Key to Electrolyte Formation: Ionization and Dissociation
- Ionization:
- Dissociation:
- Categories of Compounds that Form Electrolytes
- 1. Ionic Compounds (Salts):
- 2. Strong Acids:
- 3. Strong Bases:
- 4. Weak Acids and Weak Bases:
- 5. Certain Metal Complexes:
- 6. Molten Ionic Compounds:
- Factors Affecting Electrolyte Behavior
- Applications of Electrolytes
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
What Types of Compounds Dissolve to Become Electrolytes?
Electrolytes are substances that, when dissolved in a suitable solvent (usually water), produce a solution that can conduct electricity. This conductivity arises from the presence of freely moving ions—charged particles—within the solution. Understanding which types of compounds readily dissociate into ions and thus become electrolytes is crucial in various fields, from chemistry and biology to engineering and medicine. This comprehensive guide delves into the specifics, exploring the different classes of compounds and their behavior in solution.
The Key to Electrolyte Formation: Ionization and Dissociation
Before diving into specific compound types, let's clarify the fundamental processes: ionization and dissociation. Both lead to the formation of ions in solution, but they differ slightly in their mechanisms:
Ionization:
Ionization involves the formation of ions from neutral molecules. This often occurs when a polar solvent like water interacts with a polar or covalent compound, causing it to break apart into ions. A classic example is the ionization of a weak acid like acetic acid (CH₃COOH) in water. The water molecule's polarity helps to pull apart the hydrogen ion (H⁺) from the acetate ion (CH₃COO⁻), resulting in a solution with a limited number of ions. The process is usually reversible, meaning some of the ions can recombine to form the neutral molecule.
Dissociation:
Dissociation, on the other hand, refers to the separation of ions that already exist in a solid ionic compound. When an ionic compound, such as sodium chloride (NaCl), dissolves in water, the strong electrostatic forces holding the sodium (Na⁺) and chloride (Cl⁻) ions together are overcome by the interactions with the polar water molecules. This results in the complete separation of the ions, creating a solution rich in freely mobile charge carriers. Unlike ionization, dissociation is generally considered a complete process for strong electrolytes.
Categories of Compounds that Form Electrolytes
Several classes of compounds readily dissolve to become electrolytes. Let's examine the most important ones:
1. Ionic Compounds (Salts):
Ionic compounds, also known as salts, are formed through electrostatic attraction between positively charged cations and negatively charged anions. These compounds are typically crystalline solids at room temperature. When dissolved in a polar solvent like water, the strong ion-dipole interactions between the ions and solvent molecules overcome the electrostatic attraction holding the crystal lattice together, leading to complete dissociation of the ions. Examples include:
- Sodium chloride (NaCl): Dissolves completely into Na⁺ and Cl⁻ ions.
- Potassium nitrate (KNO₃): Dissociates into K⁺ and NO₃⁻ ions.
- Calcium sulfate (CaSO₄): While less soluble than NaCl, the portion that dissolves dissociates into Ca²⁺ and SO₄²⁻ ions.
- Magnesium bromide (MgBr₂): Dissociates into Mg²⁺ and 2Br⁻ ions.
Strong Electrolytes: Most ionic compounds are strong electrolytes, meaning they dissociate almost completely in solution, resulting in high electrical conductivity.
2. Strong Acids:
Strong acids are substances that completely ionize in water, releasing hydrogen ions (H⁺) and an anion. The high concentration of H⁺ ions makes these solutions highly acidic and excellent conductors of electricity. Examples include:
- Hydrochloric acid (HCl): Ionizes completely into H⁺ and Cl⁻.
- Sulfuric acid (H₂SO₄): Ionizes completely in two steps, yielding 2H⁺ and SO₄²⁻.
- Nitric acid (HNO₃): Ionizes completely into H⁺ and NO₃⁻.
- Perchloric acid (HClO₄): Ionizes completely into H⁺ and ClO₄⁻.
3. Strong Bases:
Strong bases are compounds that completely dissociate in water, releasing hydroxide ions (OH⁻) and a cation. These solutions are highly alkaline and exhibit high electrical conductivity due to the high concentration of OH⁻ ions. Examples include:
- Sodium hydroxide (NaOH): Dissociates completely into Na⁺ and OH⁻.
- Potassium hydroxide (KOH): Dissociates completely into K⁺ and OH⁻.
- Calcium hydroxide (Ca(OH)₂): Dissociates completely into Ca²⁺ and 2OH⁻.
- Barium hydroxide (Ba(OH)₂): Dissociates completely into Ba²⁺ and 2OH⁻.
4. Weak Acids and Weak Bases:
Unlike strong acids and bases, weak acids and bases only partially ionize in water. This means that only a small fraction of the molecules dissociate into ions, resulting in lower electrical conductivity compared to strong electrolytes. Examples include:
- Acetic acid (CH₃COOH): A weak acid that partially ionizes into CH₃COO⁻ and H⁺.
- Ammonia (NH₃): A weak base that partially reacts with water to form NH₄⁺ and OH⁻.
- Hydrofluoric acid (HF): A weak acid that partially ionizes into F⁻ and H⁺.
- Carbonic acid (H₂CO₃): A weak acid that partially ionizes in stages.
The degree of ionization for weak acids and bases is often expressed as the acid dissociation constant (Kₐ) or the base dissociation constant (Kբ). A lower Kₐ or Kբ value indicates a weaker acid or base, implying less ionization in solution.
5. Certain Metal Complexes:
Some metal complexes, especially those with highly charged metal ions and easily dissociating ligands, can act as electrolytes when dissolved in water. The extent of dissociation and hence the electrolyte behavior depends on factors like the metal ion's charge density and the nature of the ligands.
6. Molten Ionic Compounds:
Even without a solvent, molten ionic compounds can conduct electricity. In the molten state, the ions are free to move, facilitating the flow of charge. This is why molten salts are used in certain electrolytic processes.
Factors Affecting Electrolyte Behavior
Several factors influence the ability of a compound to dissolve and form an electrolyte:
- Solubility: A compound must be soluble in the solvent to form an electrolyte. Polar solvents, like water, are most effective in dissolving ionic compounds and polar molecules.
- Polarity: Polar compounds, particularly those with significant charge separation, are more likely to ionize or dissociate in polar solvents.
- Temperature: Increasing temperature generally increases solubility and the degree of ionization or dissociation, leading to enhanced conductivity.
- Concentration: The concentration of the electrolyte in the solution directly affects the conductivity. Higher concentrations usually result in higher conductivity.
- Solvent Properties: The properties of the solvent, such as its dielectric constant and polarity, significantly influence the ion-solvent interactions and therefore the degree of dissociation.
Applications of Electrolytes
Electrolytes play crucial roles in numerous applications:
- Batteries: Electrolytes are essential components of batteries, facilitating the movement of ions between the electrodes, enabling the flow of current.
- Electroplating: Electrolytes are used in electroplating processes to deposit a thin layer of metal onto a surface.
- Medicine: Electrolytes are vital for maintaining proper fluid balance and nerve function in the human body. Intravenous solutions often contain electrolytes like sodium, potassium, and chloride.
- Corrosion Prevention: Electrolytes play a role in corrosion processes. Understanding their behavior is critical for designing corrosion-resistant materials and coatings.
- Industrial Processes: Electrolytes are involved in various industrial processes, such as metal refining and water treatment.
Conclusion
The ability of a compound to dissolve and form an electrolyte hinges on its chemical structure and its interaction with the solvent. Ionic compounds, strong acids, and strong bases are the most common classes of compounds that readily form strong electrolytes, resulting in high conductivity. Weak acids and bases, on the other hand, only partially ionize, resulting in weaker electrolyte solutions. Understanding these distinctions is crucial in various scientific and technological fields, where the properties of electrolyte solutions play a pivotal role. The factors affecting electrolyte behavior, such as solubility, polarity, temperature, and solvent properties, must also be carefully considered for a comprehensive understanding.
Latest Posts
Latest Posts
-
How Many Elements Are Gases At Room Temperature
Apr 02, 2025
-
3 Main Ideas Of Cell Theory
Apr 02, 2025
-
Examples Of Liquid In Liquid Solution
Apr 02, 2025
-
Diagram Of Salt Dissolving In Water
Apr 02, 2025
-
Write The Rate Law For The Iodine Clock Reaction
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Compounds Dissolve To Become Electrolyte . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
