An Element Is Defined By The Number Of
Muz Play
Mar 25, 2025 · 6 min read
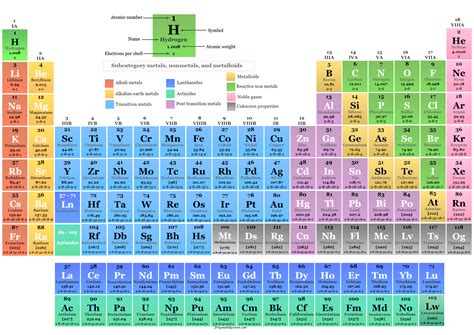
Table of Contents
An Element is Defined by the Number of Protons: A Deep Dive into Atomic Structure and the Periodic Table
The fundamental building blocks of matter are atoms, and each atom is characterized by its unique atomic structure. A critical aspect of this structure, and the defining characteristic of an element, is the number of protons within its nucleus. This number, known as the atomic number, dictates the element's identity, its chemical properties, and its position on the periodic table. This article will delve into the intricacies of atomic structure, exploring how the number of protons shapes the properties of elements and their arrangement in the periodic table.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Atoms are composed of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus.
- Neutrons: Neutral particles (no charge) also located in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells.
The nucleus, the atom's central core, contains both protons and neutrons, contributing almost all of the atom's mass. The electrons, much lighter than protons and neutrons, occupy the space surrounding the nucleus. The arrangement of these electrons determines the atom's chemical behavior.
The Significance of the Atomic Number
The atomic number, represented by the symbol Z, is the number of protons found in an atom's nucleus. This number is unique to each element and is the fundamental characteristic that defines it. For example, an atom with one proton (Z = 1) is hydrogen, an atom with two protons (Z = 2) is helium, and so on. No two elements have the same atomic number. This is the cornerstone of the periodic table's organization.
Isotopes: Variations in Neutron Number
While the number of protons defines the element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. Isotopes have the same atomic number (same number of protons) but different mass numbers (total number of protons and neutrons). For example, carbon-12 (¹²C) has six protons and six neutrons, while carbon-14 (¹⁴C) has six protons and eight neutrons. Both are isotopes of carbon, but they have different masses and, in the case of carbon-14, different stability (it's radioactive).
The Periodic Table: A Visual Representation of Element Organization
The periodic table is a tabular arrangement of chemical elements, organized by atomic number, electron configuration, and recurring chemical properties. Its organization directly reflects the number of protons in an element's atom. Elements are arranged in rows (periods) and columns (groups or families) based on their electronic structure, which is, in turn, determined by the number of protons.
Periods: Electron Shells and Energy Levels
Elements within the same period have the same number of electron shells. As you move across a period from left to right, the atomic number increases, meaning the number of protons and electrons increases. This leads to a gradual change in chemical properties within each period.
Groups: Valence Electrons and Chemical Behavior
Elements within the same group have the same number of valence electrons – electrons in the outermost electron shell. These valence electrons are primarily responsible for an element's chemical reactivity. Elements in the same group exhibit similar chemical properties due to having the same number of valence electrons, despite having different numbers of protons and electrons overall. For instance, the alkali metals (Group 1) all have one valence electron, making them highly reactive.
How the Number of Protons Influences Chemical Properties
The number of protons directly impacts the element's chemical properties in several ways:
- Electronegativity: This is the tendency of an atom to attract electrons towards itself in a chemical bond. Higher electronegativity generally indicates a stronger pull on electrons. Electronegativity is influenced by the number of protons and the distance of the valence electrons from the nucleus.
- Ionization Energy: This is the energy required to remove an electron from a neutral atom. Higher ionization energy means it's more difficult to remove an electron. Elements with a higher number of protons generally have higher ionization energy because the stronger positive charge holds the electrons more tightly.
- Reactivity: The number of valence electrons, determined by the number of protons and the element's electronic configuration, dictates the element's reactivity. Elements with nearly full or empty valence shells tend to be more reactive than those with half-filled valence shells.
- Bonding: The number of protons influences the type of chemical bonds an element can form. Elements with a high electronegativity tend to form ionic bonds, while those with lower electronegativity often form covalent bonds.
Beyond the Basics: Exploring Isotopes and Their Applications
Isotopes, as mentioned earlier, are atoms of the same element with different numbers of neutrons. While the number of protons defines the element, the different neutron numbers lead to variations in mass and nuclear stability:
Radioactive Isotopes and Their Uses
Some isotopes are radioactive, meaning their nuclei are unstable and decay over time, emitting radiation. These radioactive isotopes have numerous applications in various fields, including:
- Medical Imaging: Isotopes like technetium-99m are used in medical imaging techniques such as SPECT scans to visualize internal organs and detect abnormalities.
- Cancer Treatment: Radioactive isotopes like iodine-131 are used in radiotherapy to target and destroy cancerous cells.
- Carbon Dating: Carbon-14, a radioactive isotope of carbon, is used in carbon dating to determine the age of ancient artifacts and organic materials.
Stable Isotopes and Their Applications
Stable isotopes, unlike radioactive isotopes, do not decay. They are also utilized in various scientific and technological applications:
- Environmental Science: Stable isotope ratios are used to trace water movement, track animal migration patterns, and analyze food sources.
- Forensic Science: Stable isotope analysis can help determine the geographical origin of materials or individuals.
- Medical Research: Stable isotopes are used as tracers in metabolic studies to understand how the body processes nutrients and medications.
Conclusion: The Fundamental Role of Protons
The number of protons in an atom's nucleus is the defining characteristic of an element. This fundamental property dictates the element's atomic number, its position on the periodic table, and ultimately its chemical and physical properties. Understanding the relationship between the number of protons and the atom's structure is crucial for comprehending the behavior of matter and the vast array of chemical reactions that shape our world. From the simplest atoms to the most complex molecules, the number of protons remains the central organizing principle in the study of chemistry and the foundation of our understanding of the material universe. The continued study of atomic structure, including isotopes and their applications, will continue to reveal new insights into the intricacies of matter and its interactions, opening up new avenues for scientific discovery and technological advancement. Further research in this area will undoubtedly lead to even more innovative uses for both stable and radioactive isotopes, solidifying the importance of the number of protons in shaping our understanding of the world around us. The profound implications of this seemingly simple concept extend far beyond the classroom, impacting fields from medicine and engineering to archaeology and environmental science, showcasing the universal significance of the atomic number in determining elemental identity and behavior.
Latest Posts
Latest Posts
-
How To Do Bohr Rutherford Diagrams
May 12, 2025
-
Is Milk Pure Substance Or Mixture
May 12, 2025
-
Power Series Of 1 1 X
May 12, 2025
-
Is Boron Trifluoride Polar Or Nonpolar
May 12, 2025
-
Which Point Of The Beam Experiences The Most Compression
May 12, 2025
Related Post
Thank you for visiting our website which covers about An Element Is Defined By The Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.