An Unsaturated Solution Is One That
Muz Play
Mar 18, 2025 · 5 min read
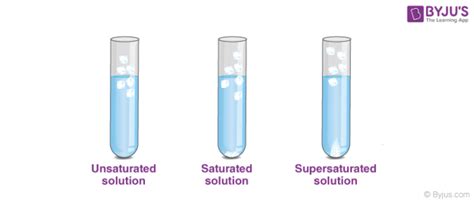
Table of Contents
An Unsaturated Solution Is One That… Can Hold More! A Deep Dive into Solution Chemistry
Understanding the properties of solutions is fundamental in chemistry and numerous applications. A crucial concept within this realm is the distinction between saturated, unsaturated, and supersaturated solutions. This article will comprehensively explore what an unsaturated solution is, detailing its characteristics, how it differs from saturated and supersaturated solutions, and its significance in various scientific and practical contexts.
Defining an Unsaturated Solution: Less is More (for now)
An unsaturated solution is a chemical solution in which the solute concentration is lower than its equilibrium solubility. In simpler terms, it means the solvent (the liquid doing the dissolving) can still dissolve more solute (the substance being dissolved) at a given temperature and pressure. Think of it like an empty swimming pool – there's plenty of room for more water (solvent) and even more swimmers (solute).
Key Characteristics of Unsaturated Solutions:
- More solute can be dissolved: This is the defining feature. Adding more solute to an unsaturated solution will result in its dissolution until the solution becomes saturated.
- Homogeneous mixture: Unsaturated solutions are homogeneous, meaning the solute is evenly distributed throughout the solvent. You won't see any solid particles settling at the bottom.
- Transparent (usually): Most unsaturated solutions are clear and transparent. The dissolved solute doesn't significantly affect the light transmission properties of the solvent.
- Dependent on temperature and pressure: The solubility of a solute in a solvent is highly dependent on temperature and, to a lesser extent, pressure. An unsaturated solution at one temperature might become saturated at a higher temperature, as higher temperatures often increase solubility.
Understanding Solubility: The Limiting Factor
To truly grasp the concept of an unsaturated solution, we must first understand solubility. Solubility is the maximum amount of a solute that can dissolve in a given amount of solvent at a specific temperature and pressure to form a stable solution. This maximum amount creates a saturated solution.
When the solute concentration exceeds the solubility limit, the excess solute begins to precipitate out of the solution, forming a solid phase. This is because the solvent molecules are already fully engaged in solvating the dissolved solute, and there's no more room for additional solute particles.
The Difference Between Unsaturated, Saturated, and Supersaturated Solutions
Let's clarify the distinctions between these three solution types:
1. Unsaturated Solution:
- Solute concentration: Less than the solubility limit.
- Appearance: Usually clear and transparent.
- Behavior upon adding more solute: More solute will dissolve.
2. Saturated Solution:
- Solute concentration: Equal to the solubility limit.
- Appearance: Usually clear, but sometimes with undissolved solute at the bottom.
- Behavior upon adding more solute: No more solute will dissolve; any additional solute will remain undissolved and precipitate out.
3. Supersaturated Solution:
- Solute concentration: Greater than the solubility limit.
- Appearance: Usually clear, but extremely unstable.
- Behavior upon adding more solute: The solution is highly unstable, and even minor disturbances (like adding a seed crystal or shaking) can cause rapid precipitation of the excess solute. This forms a saturated solution and often results in crystallization. Supersaturated solutions are usually prepared by carefully cooling a saturated solution, preventing the excess solute from precipitating.
Factors Affecting Solubility and Unsaturated Solutions
Several factors influence the solubility of a solute and, consequently, whether a solution is unsaturated, saturated, or supersaturated. These include:
1. Temperature:
Temperature significantly impacts solubility. For most solid solutes dissolved in liquid solvents, solubility increases with increasing temperature. This is because higher temperatures provide more kinetic energy to the solvent molecules, allowing them to more effectively break apart and surround the solute particles. However, there are exceptions, with some solutes exhibiting decreased solubility at higher temperatures. Gases, on the other hand, generally exhibit decreased solubility with increasing temperature.
2. Pressure:
Pressure primarily affects the solubility of gases in liquids. Increasing pressure increases the solubility of gases. This is why carbonated beverages are kept under pressure – to maintain the carbon dioxide's solubility. Pressure has a negligible effect on the solubility of most solids in liquids.
3. Nature of the Solute and Solvent:
The chemical nature of both the solute and the solvent plays a crucial role in determining solubility. The "like dissolves like" principle is commonly applied. Polar solvents (like water) tend to dissolve polar solutes (like sugar), while nonpolar solvents (like oil) tend to dissolve nonpolar solutes (like fats).
4. Particle Size:
Smaller solute particles dissolve faster than larger ones because of the increased surface area exposed to the solvent. However, particle size doesn't affect the ultimate solubility—it only influences the rate of dissolution.
Applications of Unsaturated Solutions
Unsaturated solutions are prevalent in various scientific and practical applications:
1. Medicine:
Many intravenous solutions and oral medications are formulated as unsaturated solutions to ensure rapid and complete absorption by the body.
2. Agriculture:
Fertilizers and pesticides are often prepared as unsaturated solutions to facilitate their efficient uptake by plants.
3. Industrial Processes:
Numerous industrial processes, such as electroplating and cleaning, utilize unsaturated solutions to control reaction rates and achieve desired outcomes.
4. Food and Beverage Industry:
The preparation of many beverages, such as juices and soft drinks, involves dissolving various components in water to form unsaturated solutions.
5. Environmental Science:
Understanding the solubility of pollutants in water is crucial for assessing their environmental impact and developing effective remediation strategies.
Determining Saturation: Practical Considerations
Determining whether a solution is unsaturated, saturated, or supersaturated requires careful observation and experimentation. Adding more solute is the simplest test. If more solute dissolves, the solution was unsaturated. If not, and undissolved solute remains at the bottom, the solution is saturated. Supersaturated solutions are the most unstable and often reveal themselves by spontaneous crystallization when disturbed.
Conclusion: A Foundation of Chemistry
Understanding the nature of unsaturated solutions is essential for anyone working with chemical solutions. Its ability to dissolve more solute makes it versatile in numerous applications across multiple scientific fields. The interplay of factors influencing solubility – temperature, pressure, and the chemical nature of the solute and solvent – further underscores the complexity and importance of this fundamental concept in chemistry. This comprehensive overview provides a solid foundation for further exploration of solution chemistry and its practical implications.
Latest Posts
Latest Posts
-
Mean And Variance Of Sample Mean
Mar 19, 2025
-
Predict The Product For The Reaction Shown
Mar 19, 2025
-
When Is Work Positive And Negative
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about An Unsaturated Solution Is One That . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
