Predict The Product For The Reaction Shown.
Muz Play
Mar 19, 2025 · 5 min read
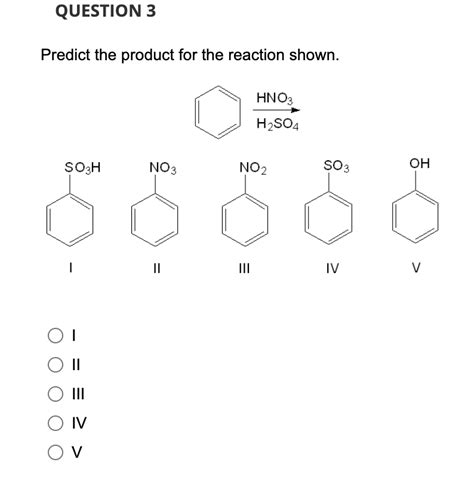
Table of Contents
Predicting the Products of Chemical Reactions: A Comprehensive Guide
Predicting the products of a chemical reaction is a fundamental skill in chemistry. It requires a solid understanding of various chemical concepts, including reaction types, reactivity series, and reaction mechanisms. This comprehensive guide will delve into various strategies and considerations for accurately predicting reaction outcomes, covering a wide range of chemical reactions.
Understanding Reaction Types: The Foundation of Prediction
Before attempting to predict products, it's crucial to identify the type of reaction. Different reaction types follow predictable patterns. Knowing the type significantly narrows down the possibilities. Common reaction types include:
1. Combination (Synthesis) Reactions:
Definition: Two or more reactants combine to form a single product.
General Form: A + B → AB
Example: 2Na(s) + Cl₂(g) → 2NaCl(s) (Sodium and chlorine react to form sodium chloride)
Prediction: Identify the elements or compounds combining and predict the resulting compound, considering valencies and charges to ensure a balanced formula.
2. Decomposition Reactions:
Definition: A single reactant breaks down into two or more simpler products. Often requires energy input (heat, light, electricity).
General Form: AB → A + B
Example: 2H₂O(l) → 2H₂(g) + O₂(g) (Water decomposes into hydrogen and oxygen upon electrolysis)
Prediction: Consider the stability of the reactant. Less stable compounds are more prone to decomposition. Products often reflect the constituent elements or simpler compounds.
3. Single Displacement (Substitution) Reactions:
Definition: One element replaces another in a compound. Reactivity series is crucial for prediction.
General Form: A + BC → AC + B
Example: Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s) (Zinc displaces copper from copper sulfate)
Prediction: Refer to the reactivity series. A more reactive element will displace a less reactive element.
4. Double Displacement (Metathesis) Reactions:
Definition: Two compounds exchange ions to form two new compounds. Often involves precipitation, gas evolution, or water formation.
General Form: AB + CD → AD + CB
Example: AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq) (Silver nitrate and sodium chloride react to form silver chloride precipitate and sodium nitrate)
Prediction: Consider solubility rules to predict precipitate formation. Look for gas evolution (e.g., CO₂, SO₂) or neutralization (acid-base reaction) leading to water formation.
5. Combustion Reactions:
Definition: A substance reacts rapidly with oxygen, producing heat and light. Often involves hydrocarbons reacting with oxygen to produce carbon dioxide and water.
General Form: CxHy + O₂ → CO₂ + H₂O
Example: CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g) (Methane combustion)
Prediction: For hydrocarbons, predict CO₂ and H₂O as products. Incomplete combustion may yield CO or C (soot).
6. Acid-Base Reactions (Neutralization):
Definition: An acid reacts with a base to form salt and water.
General Form: HA + BOH → BA + H₂O
Example: HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l) (Hydrochloric acid and sodium hydroxide react to form sodium chloride and water)
Prediction: The salt formed is determined by the cation from the base and the anion from the acid.
Advanced Considerations for Accurate Predictions
Predicting reaction products often requires going beyond simple reaction type identification. Several factors play crucial roles:
1. Reaction Conditions:
Temperature, pressure, presence of catalysts, and the concentration of reactants significantly influence the reaction pathway and the products formed. For example, the combustion of methane can produce different products under different oxygen levels.
2. Reactivity Series:
The reactivity series provides a relative order of reactivity for metals and non-metals. This series is essential in predicting single displacement reactions. A more reactive metal will displace a less reactive metal from its compound.
3. Solubility Rules:
Solubility rules are crucial for predicting the formation of precipitates in double displacement reactions. These rules describe the solubility of various ionic compounds in water.
4. Oxidation-Reduction (Redox) Reactions:
Redox reactions involve the transfer of electrons. Identifying the oxidizing and reducing agents helps predict the oxidation states of products. Changes in oxidation states indicate electron transfer.
5. Reaction Mechanisms:
Understanding the reaction mechanism, the step-by-step process of a reaction, provides detailed insight into the formation of products. This is especially crucial for complex organic reactions.
6. Equilibrium Considerations:
Many reactions are reversible, reaching an equilibrium state where the rates of the forward and reverse reactions are equal. The equilibrium constant (K) determines the relative amounts of reactants and products at equilibrium.
7. Kinetic Factors:
The rate of a reaction influences the product distribution. Reactions with high activation energies may proceed slowly, while others might be fast, leading to different product formations depending on the reaction time.
Practical Examples and Detailed Predictions
Let's analyze some examples to illustrate how to apply these principles:
Example 1: Predict the products of the reaction between hydrochloric acid (HCl) and magnesium (Mg).
- Reaction Type: Single displacement
- Reactivity Series: Magnesium is more reactive than hydrogen.
- Prediction: Magnesium will displace hydrogen from hydrochloric acid, forming magnesium chloride and hydrogen gas.
- Balanced Equation: Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
Example 2: Predict the products of the reaction between sodium hydroxide (NaOH) and sulfuric acid (H₂SO₄).
- Reaction Type: Acid-base neutralization
- Prediction: The reaction will produce sodium sulfate and water.
- Balanced Equation: 2NaOH(aq) + H₂SO₄(aq) → Na₂SO₄(aq) + 2H₂O(l)
Example 3: Predict the products of the reaction between lead(II) nitrate (Pb(NO₃)₂) and potassium iodide (KI).
- Reaction Type: Double displacement
- Solubility Rules: Lead(II) iodide (PbI₂) is insoluble.
- Prediction: A precipitate of lead(II) iodide will form, along with potassium nitrate in solution.
- Balanced Equation: Pb(NO₃)₂(aq) + 2KI(aq) → PbI₂(s) + 2KNO₃(aq)
Example 4: Predict the products of the combustion of propane (C₃H₈) in excess oxygen.
- Reaction Type: Combustion
- Prediction: Carbon dioxide and water will be the products.
- Balanced Equation: C₃H₈(g) + 5O₂(g) → 3CO₂(g) + 4H₂O(g)
Conclusion: Mastering the Art of Prediction
Predicting the products of chemical reactions is a multifaceted skill requiring a strong foundational knowledge of chemical concepts and principles. By carefully considering the reaction type, reaction conditions, reactivity series, solubility rules, and other relevant factors, you can significantly improve your ability to accurately predict the outcomes of chemical reactions. Practice is key; the more reactions you analyze, the better you'll become at recognizing patterns and applying your knowledge to new situations. Remember, understanding the underlying principles is more important than memorizing specific reactions. This understanding will enable you to approach any chemical reaction with confidence and accurately predict its products.
Latest Posts
Latest Posts
-
What Is Zone Of Inhibition In Microbiology
Mar 19, 2025
-
How To Add Formal Charges To Resonance Structures
Mar 19, 2025
-
What Are The Building Blocks Of A Carbohydrate
Mar 19, 2025
-
Substitution And Elimination Reactions Practice Problems
Mar 19, 2025
-
The Permanent Party Organization Consists Of
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Predict The Product For The Reaction Shown. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
