What Is Zone Of Inhibition In Microbiology
Muz Play
Mar 19, 2025 · 7 min read
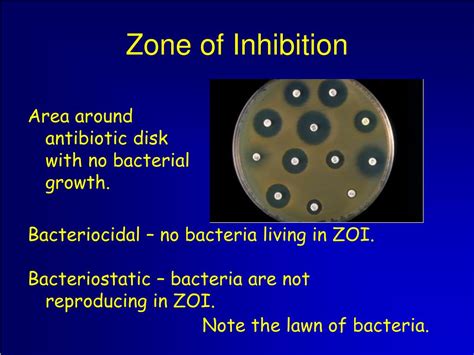
Table of Contents
What is the Zone of Inhibition in Microbiology? A Comprehensive Guide
The zone of inhibition (ZOI) is a crucial concept in microbiology, representing a powerful tool for evaluating the effectiveness of antimicrobial agents against various microorganisms. Understanding the ZOI, its measurement, interpretation, and limitations is essential for researchers, clinicians, and anyone involved in studying antimicrobial susceptibility. This comprehensive guide delves deep into the intricacies of the zone of inhibition, providing a thorough understanding of its significance in the field of microbiology.
Understanding the Fundamentals of the Zone of Inhibition
The zone of inhibition is the clear area surrounding an antimicrobial agent disk on an agar plate where bacterial growth has been inhibited. This inhibition is a visual representation of the antimicrobial's effectiveness against the tested microorganism. The larger the ZOI, the more potent the antimicrobial agent is considered against that specific microbe. The principle behind this technique rests on the diffusion of the antimicrobial agent from the disk into the surrounding agar. The concentration gradient created allows for the observation of microbial growth inhibition at different distances from the disk.
How is the Zone of Inhibition Formed?
The formation of the ZOI is a dynamic process driven by several factors:
-
Diffusion: The antimicrobial agent diffuses from the disk into the surrounding agar medium. The rate of diffusion varies depending on the properties of the agent (size, solubility, charge) and the agar itself.
-
Concentration Gradient: A concentration gradient is established, with the highest concentration of the agent directly surrounding the disk and gradually decreasing further away.
-
Minimum Inhibitory Concentration (MIC): The MIC is the lowest concentration of an antimicrobial agent that prevents visible growth of a specific microorganism after a period of incubation. The edge of the ZOI generally corresponds to the area where the antimicrobial concentration falls below the MIC.
-
Microbial Growth Rate: The growth rate of the tested microorganism influences the clarity and size of the ZOI. Faster-growing microorganisms will often show a sharper transition between the inhibited zone and the area of growth.
Methods for Determining the Zone of Inhibition
The Kirby-Bauer disk diffusion test is the most widely used method for determining the zone of inhibition. This standardized technique allows for consistent and comparable results across different laboratories. The steps involved include:
-
Preparing the inoculum: A standardized suspension of the test microorganism is prepared to ensure a consistent number of bacterial cells are present on the agar plate.
-
Preparing the agar plate: A Mueller-Hinton agar plate is typically used due to its consistent composition and suitability for antimicrobial diffusion. The agar is evenly inoculated with the bacterial suspension.
-
Applying the antimicrobial disks: Sterile disks impregnated with known concentrations of different antimicrobial agents are placed onto the agar surface.
-
Incubating the plate: The inoculated plate is incubated under controlled conditions (temperature, time, and atmosphere) to allow for bacterial growth and antimicrobial diffusion.
-
Measuring the Zone of Inhibition: After incubation, the diameter of the clear zone surrounding each disk is measured in millimeters. These measurements are then compared to established interpretive standards to determine the susceptibility or resistance of the microorganism to the tested antimicrobial agents.
Importance of Standardization
The accuracy and reliability of the ZOI measurement are critically dependent on standardization. This includes:
-
Using standardized media: Mueller-Hinton agar is recommended for its consistent composition and suitability for antimicrobial diffusion.
-
Using standardized inoculum: The concentration of bacteria in the inoculum should be standardized to ensure consistent results.
-
Using standardized incubation conditions: Incubation temperature, time, and atmosphere must be strictly controlled to ensure consistent diffusion and growth.
-
Accurate measurement: The ZOI should be measured accurately, typically using a ruler or caliper, to ensure reliable results.
Failure to adhere to these standardized procedures can lead to inaccurate ZOI measurements and misinterpretations of antimicrobial susceptibility.
Interpreting the Zone of Inhibition Results
Once the ZOI diameter is measured, it is compared to established interpretive standards provided by organizations like the Clinical and Laboratory Standards Institute (CLSI). These standards define the diameter ranges corresponding to susceptible, intermediate, or resistant categories.
-
Susceptible: A large ZOI indicates the microorganism is susceptible to the antimicrobial agent, suggesting that the agent can effectively inhibit its growth at clinically achievable concentrations.
-
Intermediate: An intermediate ZOI indicates that the microorganism's response to the antimicrobial agent is uncertain. Higher concentrations of the agent may be needed for effective treatment.
-
Resistant: A small or absent ZOI suggests the microorganism is resistant to the antimicrobial agent, indicating that the agent is unlikely to be effective in treating the infection.
Factors Affecting ZOI Interpretation
Several factors can influence the interpretation of ZOI results:
-
Antimicrobial properties: The physicochemical properties of the antimicrobial agent (e.g., solubility, diffusion rate) affect its ability to diffuse through the agar and create a ZOI.
-
Microbial properties: The inherent properties of the microorganism, including its growth rate and susceptibility to the antimicrobial agent, significantly influence ZOI size.
-
Technical factors: Inconsistent techniques, such as variations in inoculum density or incubation conditions, can affect the ZOI and its interpretation.
-
Bacterial interactions: The presence of other microorganisms in the sample might affect the ZOI by competing for resources or producing substances that interfere with the antimicrobial agent.
Beyond the Kirby-Bauer Test: Advanced Techniques
While the Kirby-Bauer test is a widely used and valuable technique, other methods provide more precise information on antimicrobial susceptibility. These include:
-
Minimum Inhibitory Concentration (MIC) determination: MIC testing precisely determines the lowest concentration of an antimicrobial agent that inhibits visible growth of a microorganism. This method provides a more quantitative assessment of antimicrobial susceptibility compared to the ZOI measurement. Common methods include broth microdilution and Etest.
-
Minimum Bactericidal Concentration (MBC) determination: MBC testing determines the lowest concentration of an antimicrobial agent that kills a specified proportion of the microorganisms. This method provides information on the killing capacity of the agent, which is crucial for treating severe infections.
-
Time-kill assays: These assays provide detailed kinetics of antimicrobial activity over time, offering a more comprehensive understanding of the antimicrobial agent's effects on the microorganism's population.
The Importance of the Zone of Inhibition in Clinical Practice
The zone of inhibition plays a vital role in clinical microbiology laboratories. It serves as a crucial tool for:
-
Guiding antimicrobial therapy: ZOI results help clinicians select appropriate antimicrobial agents for treating bacterial infections based on the susceptibility profile of the isolated pathogen.
-
Monitoring antimicrobial resistance: Tracking changes in ZOI over time can help monitor the emergence and spread of antimicrobial resistance within a community or hospital.
-
Evaluating new antimicrobial agents: The ZOI method can be used to evaluate the efficacy of novel antimicrobial compounds during the drug discovery and development process.
Limitations of the Zone of Inhibition Method
While the ZOI is a valuable tool, it does have limitations:
-
Qualitative nature: The ZOI provides a qualitative assessment of antimicrobial susceptibility, rather than a precise quantitative measurement like the MIC.
-
Influence of factors: The ZOI is susceptible to various factors, including the inoculum density, incubation conditions, and bacterial interactions, which can affect the accuracy of the results.
-
Limited information: The ZOI provides limited information about the mechanism of antimicrobial action and the bactericidal or bacteriostatic nature of the agent.
Conclusion
The zone of inhibition is a fundamental concept in microbiology, representing a valuable tool for assessing antimicrobial susceptibility. Its simplicity, affordability, and wide applicability make it a cornerstone of clinical microbiology laboratories and research settings. While limitations exist, understanding its principles, methods, and interpretations, combined with the use of more precise techniques like MIC determination, provides a comprehensive approach to evaluating antimicrobial activity and combating the ever-growing challenge of antimicrobial resistance. Continued research and development of novel antimicrobial agents are crucial to address the increasing threat of drug-resistant microorganisms and ensure the effective treatment of infectious diseases. The zone of inhibition will undoubtedly remain an important component of this ongoing effort.
Latest Posts
Latest Posts
-
Using Inverse Matrix To Solve System Of Equations
Mar 19, 2025
-
How Does Osmosis Affect Animal Cells
Mar 19, 2025
-
Algebra 1 Factor The Common Factor Out Of Each Expression
Mar 19, 2025
-
Weak Acid And Weak Base Ph
Mar 19, 2025
-
Is There A Keyboard Corelation To Find Periodic Table Fonts
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is Zone Of Inhibition In Microbiology . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
