Weak Acid And Weak Base Ph
Muz Play
Mar 19, 2025 · 6 min read
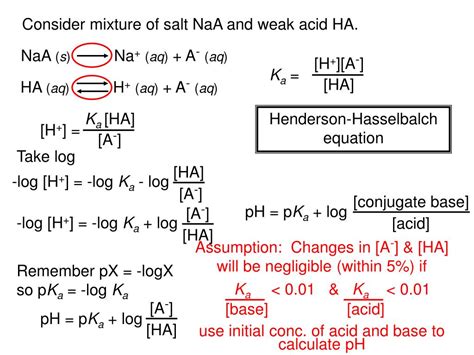
Table of Contents
Weak Acid and Weak Base pH: A Comprehensive Guide
Understanding pH is crucial in various fields, from chemistry and biology to environmental science and medicine. This comprehensive guide delves into the intricacies of weak acid and weak base pH calculations, providing a clear and detailed explanation for both beginners and those seeking a deeper understanding. We'll explore the underlying principles, relevant equations, and practical applications, equipping you with the knowledge to confidently tackle pH-related problems.
Understanding pH: A Quick Recap
Before diving into weak acids and bases, let's briefly review the concept of pH. pH is a measure of the acidity or basicity (alkalinity) of a solution. It's based on the concentration of hydrogen ions (H⁺) in the solution, with a scale ranging from 0 to 14:
- pH 0-7: Acidic solution (lower pH indicates stronger acidity)
- pH 7: Neutral solution
- pH 7-14: Basic (alkaline) solution (higher pH indicates stronger basicity)
The pH is calculated using the following formula:
pH = -log₁₀[H⁺]
where [H⁺] represents the concentration of hydrogen ions in moles per liter (mol/L).
Weak Acids and Their Dissociation
Unlike strong acids (like HCl or HNO₃) which completely dissociate in water, weak acids only partially dissociate. This means that only a small fraction of the acid molecules donate a proton (H⁺) to water molecules. The equilibrium reaction for a generic weak acid (HA) is:
HA(aq) ⇌ H⁺(aq) + A⁻(aq)
The equilibrium constant for this reaction is called the acid dissociation constant (Ka):
Ka = [H⁺][A⁻]/[HA]
A smaller Ka value indicates a weaker acid; a larger Ka value indicates a stronger weak acid.
Calculating the pH of a Weak Acid Solution
Calculating the pH of a weak acid solution involves using the Ka value and the initial concentration of the acid. We can use an ICE (Initial, Change, Equilibrium) table to solve for the equilibrium concentrations of H⁺, A⁻, and HA. This table is crucial for setting up the calculation correctly.
Let's consider a specific example: A 0.1 M solution of acetic acid (CH₃COOH) has a Ka value of 1.8 x 10⁻⁵.
| Species | Initial (M) | Change (M) | Equilibrium (M) |
|---|---|---|---|
| CH₃COOH | 0.1 | -x | 0.1 - x |
| H⁺ | 0 | +x | x |
| CH₃COO⁻ | 0 | +x | x |
Substituting these equilibrium concentrations into the Ka expression:
1.8 x 10⁻⁵ = x²/(0.1 - x)
Since Ka is small, we can often assume that x is negligible compared to 0.1 (the 5% rule). This simplifies the equation to:
1.8 x 10⁻⁵ ≈ x²/0.1
Solving for x (which represents [H⁺]):
x = √(1.8 x 10⁻⁶) ≈ 1.34 x 10⁻³ M
Therefore, the pH is:
pH = -log₁₀(1.34 x 10⁻³) ≈ 2.87
Weak Bases and Their Dissociation
Similar to weak acids, weak bases only partially dissociate in water. A generic weak base (B) reacts with water to form hydroxide ions (OH⁻):
B(aq) + H₂O(l) ⇌ BH⁺(aq) + OH⁻(aq)
The equilibrium constant for this reaction is called the base dissociation constant (Kb):
Kb = [BH⁺][OH⁻]/[B]
A smaller Kb value indicates a weaker base, and a larger Kb value indicates a stronger weak base.
Calculating the pH of a Weak Base Solution
The calculation of the pH of a weak base solution is analogous to that of a weak acid, but with some key differences. We use the Kb value and the ICE table to determine the equilibrium concentration of OH⁻. Then, we use the relationship between [H⁺] and [OH⁻] in water at 25°C:
Kw = [H⁺][OH⁻] = 1.0 x 10⁻¹⁴
where Kw is the ion product constant of water.
Let's illustrate with an example: A 0.05 M solution of ammonia (NH₃) has a Kb value of 1.8 x 10⁻⁵. Following the same ICE table method as before, we can solve for [OH⁻], then calculate [H⁺] using Kw, and finally calculate the pH. The steps are similar to the weak acid calculation but use Kb instead of Ka.
The Relationship Between Ka and Kb
For conjugate acid-base pairs, there's a direct relationship between Ka and Kb:
Ka x Kb = Kw = 1.0 x 10⁻¹⁴
This equation is extremely useful when you know either Ka or Kb for a conjugate pair and need to calculate the other.
Buffers and pH Control
Buffers are solutions that resist changes in pH upon addition of small amounts of acid or base. They typically consist of a weak acid and its conjugate base (or a weak base and its conjugate acid). The Henderson-Hasselbalch equation is used to calculate the pH of a buffer solution:
pH = pKa + log₁₀([A⁻]/[HA])
where pKa = -log₁₀(Ka). This equation is particularly helpful in understanding how the ratio of the conjugate base to weak acid affects the buffer's pH.
Polyprotic Acids and Bases
Polyprotic acids are acids that can donate more than one proton (e.g., H₂SO₄, H₃PO₄). Calculating the pH of a polyprotic acid solution requires considering the stepwise dissociation constants (Ka1, Ka2, etc.). Similarly, polyprotic bases can accept more than one proton. Each dissociation step has its own equilibrium constant.
Factors Affecting Weak Acid and Weak Base pH
Several factors influence the pH of weak acid and weak base solutions:
- Concentration: Higher concentration leads to a lower pH for weak acids and a higher pH for weak bases.
- Temperature: Temperature affects the equilibrium constants (Ka and Kb), thus impacting the pH.
- Presence of other ions: The presence of common ions (from salts) can affect the pH through the common ion effect.
Practical Applications of Weak Acid and Weak Base pH
Understanding weak acid and weak base pH is crucial in many applications:
- Medicine: Many biological systems rely on buffers to maintain a stable pH. This is critical for enzyme function and overall physiological processes.
- Environmental Science: pH plays a significant role in water quality and ecosystem health. Understanding weak acid/base equilibria is important for analyzing water samples and assessing environmental impact.
- Agriculture: Soil pH is vital for plant growth. Farmers often use buffers and pH adjustments to optimize growing conditions.
- Industrial Processes: Many industrial processes involve pH control using weak acids and bases to maintain optimal reaction conditions.
Advanced Topics and Further Exploration
This guide provides a foundation for understanding weak acid and weak base pH. For more advanced studies, consider exploring these topics:
- Titration curves: These curves illustrate the pH changes during the titration of a weak acid or base with a strong base or acid.
- Activity coefficients: These corrections account for the non-ideal behavior of ions in concentrated solutions.
- More complex buffer systems: Exploring buffer systems with multiple weak acids or bases.
By mastering the concepts presented here, you'll gain a strong understanding of weak acid and weak base pH calculations and their vast applications across various scientific disciplines. Remember to practice solving problems to solidify your understanding and build your confidence in tackling more complex scenarios. The more you practice, the easier it will become to navigate the intricacies of pH calculations.
Latest Posts
Latest Posts
-
What Is The Characteristics Of Population
Mar 19, 2025
-
What Years Were Buffalo Nickels Made
Mar 19, 2025
-
Solving Systems Of Linear Equations By Substitution Answer Key
Mar 19, 2025
-
What Is The Basic Functional Unit Of The Kidney
Mar 19, 2025
-
Membrane Proteins That Create A Watertight Seal
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Weak Acid And Weak Base Ph . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
