Membrane Proteins That Create A Watertight Seal
Muz Play
Mar 19, 2025 · 6 min read
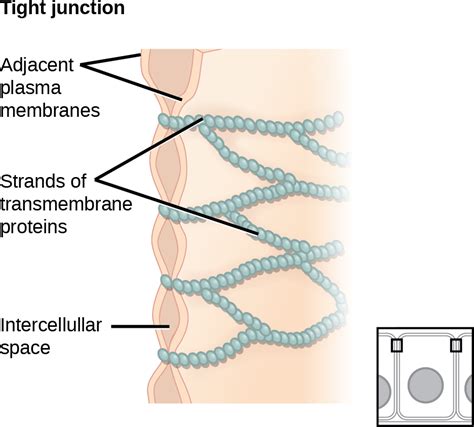
Table of Contents
Membrane Proteins That Create a Watertight Seal: A Deep Dive into Tight Junctions and Cell Adhesion
Tight junctions (TJs) are specialized structures found in epithelial and endothelial cells that form a watertight seal between adjacent cells. This seal is crucial for maintaining the integrity of tissues and organs, preventing the leakage of fluids and molecules between cells. The formation of this impermeable barrier relies on a complex interplay of transmembrane proteins, associated cytoplasmic proteins, and the cytoskeleton. Understanding the intricacies of these proteins is critical to comprehending various physiological processes and pathological conditions. This article delves into the key membrane proteins involved in creating this vital watertight seal, exploring their structure, function, and the implications of their dysfunction.
The Key Players: Transmembrane Proteins of Tight Junctions
Several transmembrane proteins are essential components of tight junctions, each playing a distinct role in establishing and maintaining the seal. Among the most important are the claudins, occludin, and junctional adhesion molecules (JAMs).
Claudins: The Master Architects of the Tight Junction Seal
Claudins are a family of tetraspan transmembrane proteins that represent the core structural components of TJs. To date, over 27 different claudin genes have been identified in mammals, each encoding a protein with unique properties and tissue-specific expression patterns. This diversity allows for the fine-tuning of TJ permeability in different tissues and organs. For instance, some claudins contribute to the formation of highly selective paracellular barriers, while others permit the passage of specific ions or small molecules.
The structure of a claudin molecule is characterized by four transmembrane domains, two extracellular loops (EC1 and EC2), and cytoplasmic N- and C-termini. The extracellular loops, particularly EC1 and EC2, are crucial for mediating homophilic and heterophilic interactions between claudins on adjacent cells. These interactions are essential for the formation of the tight junction strand network, the physical basis of the barrier function. The cytoplasmic domains interact with various cytoplasmic proteins, linking the TJ to the actin cytoskeleton and regulating its dynamics.
Different claudins exhibit varying degrees of charge selectivity. Some claudins are cation-selective, favoring the passage of positively charged ions, while others are anion-selective, favoring negatively charged ions. Still others are less selective, allowing the passage of both cations and anions. This diversity in charge selectivity ensures the precise regulation of ion and solute transport across epithelial and endothelial barriers.
Occludin: A Modulator of Tight Junction Function
Occludin, another transmembrane protein, plays a more ambiguous role in TJ function. While it contributes to the structural integrity of the junction, its role in barrier formation is less clear than that of claudins. Occludin’s four transmembrane domains and extracellular loops interact with claudins and other TJ proteins, but its exact contribution to barrier function is still under investigation.
Some studies suggest that occludin plays a more significant role in regulating TJ dynamics and cell signaling rather than directly contributing to the barrier's impermeability. Its interaction with various intracellular proteins suggests a role in cell signaling pathways impacting TJ organization and function. Unlike claudins, occludin knockout mice do not display severely impaired barrier function, suggesting a degree of functional redundancy or compensation by other TJ proteins.
Junctional Adhesion Molecules (JAMs): Linking Cell Adhesion and Tight Junctions
JAMs are immunoglobulin superfamily members that contribute to both cell adhesion and tight junction formation. They are integral membrane proteins with a single transmembrane domain and extracellular immunoglobulin-like domains. JAMs are involved in various cellular processes, including leukocyte transmigration across endothelial barriers and the regulation of TJ permeability.
JAMs interact with both claudins and occludin, contributing to the structural organization of the TJ complex. Moreover, they play a vital role in regulating the permeability of the paracellular pathway, mediating interactions with leukocytes and other cells. Different JAM isoforms exhibit tissue-specific expression and distinct functional roles, further highlighting the complexity of TJ regulation.
Beyond the Transmembrane Proteins: The Cytoplasmic Network
The transmembrane proteins of the tight junction don't function in isolation. Their cytoplasmic domains interact with a complex network of cytoplasmic scaffolding proteins that anchor the TJs to the actin cytoskeleton and regulate their dynamics. These proteins include ZO-1, ZO-2, ZO-3, cingulin, and others.
ZO Proteins: Key Cytoplasmic Anchors
The zonula occludens (ZO) proteins (ZO-1, ZO-2, and ZO-3) are a family of membrane-associated guanylate kinases (MAGUKs) that play a crucial role in anchoring the TJ to the actin cytoskeleton. They interact directly with the cytoplasmic tails of claudins and occludin, linking the TJ complex to the actin filaments. This linkage is essential for maintaining the structural integrity and stability of the TJ. The ZO proteins also act as scaffolding proteins, interacting with other signaling molecules and regulating the assembly and disassembly of the junction.
Cingulin and Other Cytoplasmic Proteins
Cingulin is another important cytoplasmic protein associated with TJs. It is thought to play a role in regulating the organization and dynamics of the TJ strands. The exact mechanisms by which cingulin achieves this regulation are still being investigated, but it is believed to interact with both ZO proteins and actin filaments.
Other cytoplasmic proteins associated with TJs include symplekin, and various signaling molecules. These proteins contribute to the regulation of TJ assembly, maintenance, and function.
The Importance of the Watertight Seal: Physiological Implications
The watertight seal created by tight junctions is crucial for numerous physiological processes:
-
Maintaining tissue homeostasis: TJs prevent the uncontrolled passage of fluids, ions, and macromolecules between cells, maintaining the integrity and proper function of tissues and organs. This is particularly crucial in the intestines, kidneys, and blood-brain barrier.
-
Regulating paracellular transport: TJs selectively control the passage of ions and small molecules across the paracellular pathway, influencing the absorption and secretion of substances in various organs.
-
Preventing pathogen invasion: The tight junctions act as a physical barrier against the invasion of pathogens. A compromised TJ barrier can lead to increased susceptibility to infections.
-
Maintaining tissue polarity: TJs help maintain the apical-basolateral polarity of epithelial and endothelial cells, which is essential for their specialized functions.
-
Wound healing: TJs play a role in the process of wound healing, contributing to the restoration of tissue integrity.
Dysfunction of Tight Junctions: Pathological Consequences
Disruption of tight junction integrity can lead to a variety of pathological conditions:
-
Inflammatory bowel disease (IBD): Compromised TJ function is a hallmark of IBD, contributing to increased intestinal permeability and inflammation.
-
Infectious diseases: Disruption of TJs allows pathogens to invade tissues and cause infection.
-
Cancer: Loss of TJ function can contribute to cancer progression and metastasis.
-
Autoimmune diseases: Dysregulation of TJ function is implicated in several autoimmune diseases, contributing to inflammation and tissue damage.
-
Neurological disorders: Disruptions in the blood-brain barrier, which relies on tight junctions, contribute to neurological disorders.
Future Directions and Research
Despite significant advancements in our understanding of tight junctions, many questions remain. Future research will likely focus on:
-
Further elucidation of the intricate interactions between different TJ proteins and their regulatory mechanisms.
-
Developing novel therapeutic strategies targeting TJ proteins to treat diseases associated with TJ dysfunction.
-
A more thorough understanding of the role of post-translational modifications in regulating TJ function.
-
Exploring the complex interplay between the TJ and the surrounding cell environment.
In conclusion, the watertight seal formed by tight junctions is a sophisticated and highly regulated process involving a complex interplay of transmembrane proteins, cytoplasmic proteins, and the cytoskeleton. Understanding these intricate interactions is crucial for appreciating the physiological roles of TJs and the pathological consequences of their dysfunction. Future research in this area will continue to reveal new insights into this fascinating and essential aspect of cellular biology.
Latest Posts
Latest Posts
-
What Step In Photosynthesis May Occur During Day And Night
Mar 19, 2025
-
Subatomic Particle With A Neutral Charge
Mar 19, 2025
-
Sampling Distribution Of The Mean Calculator
Mar 19, 2025
-
Ce Qui Ce Que Ce Dont
Mar 19, 2025
-
Lab Report Titration Of Acids And Bases
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Membrane Proteins That Create A Watertight Seal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
