How Does Osmosis Affect Animal Cells
Muz Play
Mar 19, 2025 · 6 min read
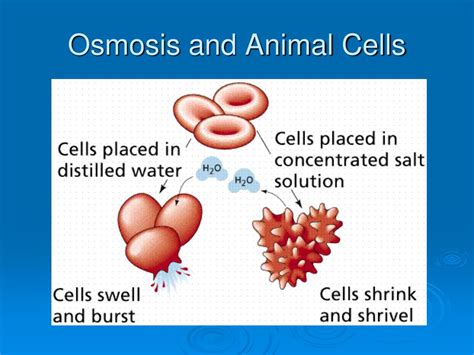
Table of Contents
How Does Osmosis Affect Animal Cells? A Comprehensive Guide
Osmosis, the passive movement of water across a selectively permeable membrane from a region of high water concentration to a region of low water concentration, is a fundamental process impacting all living organisms, especially animal cells. Understanding how osmosis affects animal cells is crucial to comprehending various physiological processes, from nutrient absorption to waste excretion, and even the development of certain diseases. This comprehensive guide delves deep into the mechanics of osmosis in animal cells, exploring its effects under different osmotic conditions and highlighting its significance in maintaining cellular homeostasis.
Understanding Osmosis: The Basics
Before diving into the intricacies of osmosis in animal cells, let's solidify our understanding of the core principles. Osmosis occurs due to the difference in water potential between two solutions separated by a semi-permeable membrane. This membrane allows water molecules to pass through but restricts the movement of larger solute molecules. Water moves to equalize the water potential, striving for equilibrium.
This movement is driven by the tendency of water to move from an area of high water potential (low solute concentration) to an area of low water potential (high solute concentration). Think of it like this: if you have a container separated by a selectively permeable membrane, and one side has pure water while the other has a salty solution, water will move from the pure water side to the salty side to dilute the salt.
Key terms to remember:
- Hypertonic Solution: A solution with a higher solute concentration than the cell's cytoplasm.
- Hypotonic Solution: A solution with a lower solute concentration than the cell's cytoplasm.
- Isotonic Solution: A solution with the same solute concentration as the cell's cytoplasm.
- Water Potential: The tendency of water to move from one area to another.
Osmosis in Animal Cells: The Impact of Different Environments
The behavior of an animal cell immersed in a solution depends heavily on the tonicity of that solution. Let's explore the effects of each type of solution:
1. Hypotonic Solution: Swelling and Lysis
When an animal cell is placed in a hypotonic solution, the water potential outside the cell is higher than inside. Consequently, water rushes into the cell across the cell membrane via osmosis. This influx of water causes the cell to swell. Because animal cells lack a rigid cell wall (unlike plant cells), excessive swelling can lead to lysis, or the bursting of the cell. This is because the cell membrane is unable to withstand the increased internal pressure. Lysis is a detrimental event, potentially leading to cell death and disrupting tissue function.
2. Hypertonic Solution: Shrinkage and Crenation
In a hypertonic solution, the water potential outside the cell is lower than inside. This means the concentration of solutes outside the cell is higher. As a result, water moves out of the animal cell by osmosis. This outflow of water causes the cell to shrink and shrivel, a process known as crenation. Crenation alters cell shape and function, potentially leading to cell death if the water loss is significant. The cell membrane pulls away from the cell wall in plant cells, but in animal cells, the entire cell shrinks.
3. Isotonic Solution: Dynamic Equilibrium
An isotonic solution has the same water potential as the animal cell's cytoplasm. In this environment, there is no net movement of water across the cell membrane. Water continues to move in and out, but the rates are equal, maintaining a state of dynamic equilibrium. This is generally the ideal condition for animal cells as it prevents both excessive swelling and shrinkage, ensuring optimal cell function.
Osmosis and Cellular Processes: Examples in Animal Systems
Osmosis isn't just a theoretical concept; it plays a crucial role in numerous physiological processes in animals. Here are some examples:
1. Nutrient Absorption in the Digestive System
The absorption of water and essential nutrients in the digestive tract relies heavily on osmosis. As digested food moves through the intestines, the concentration of nutrients increases in the lumen (the interior space of the intestines). This creates a concentration gradient, driving the osmosis of water from the intestinal cells into the lumen, facilitating the absorption of nutrients into the bloodstream. This is a carefully regulated process, ensuring efficient nutrient uptake without causing harm to the intestinal cells.
2. Kidney Function and Waste Excretion
The kidneys play a critical role in maintaining water balance in the body. Through a complex process of filtration and reabsorption, the kidneys regulate the concentration of solutes in the blood. Osmosis plays a vital role in this process. As blood passes through the nephrons (the functional units of the kidney), water is reabsorbed into the bloodstream, preventing excessive water loss in urine. The concentration gradients created by the kidneys ensure the efficient removal of waste products while maintaining optimal hydration.
3. Cell Signaling and Communication
Osmosis indirectly influences cell signaling pathways. Changes in cell volume due to osmotic shifts can activate mechanosensitive ion channels in the cell membrane. These channels, when activated, can trigger intracellular signaling cascades, leading to changes in gene expression and cellular behavior. This highlights the interconnectedness of osmosis with other fundamental cellular processes.
4. Osmosis and Disease
Disruptions in osmotic balance can lead to various health issues. For example, dehydration, a condition characterized by excessive water loss, results in a hypertonic environment in the body's cells. This leads to cellular shrinkage and impaired function, impacting various organ systems. Conversely, water intoxication, resulting from excessive water intake, creates a hypotonic environment, potentially causing cellular swelling and lysis, especially in brain cells which can be fatal. Certain kidney diseases also disrupt the body's ability to regulate osmotic balance, leading to serious health complications.
Maintaining Osmotic Balance: Homeostasis and Cellular Regulation
Maintaining proper osmotic balance is essential for the survival and function of animal cells. This balance, known as homeostasis, is achieved through various regulatory mechanisms. These mechanisms work to counteract changes in extracellular osmolarity and maintain a stable intracellular environment.
For instance, cells possess sophisticated transport mechanisms that help them regulate the movement of solutes in and out. These include various ion pumps and channels that actively transport ions across the cell membrane, counteracting osmotic forces. The kidneys also play a crucial role in regulating body fluid osmolarity, ensuring that the extracellular environment remains within a narrow range of osmotic pressure.
Conclusion: The Significance of Osmosis in Animal Biology
Osmosis is a fundamental process with far-reaching consequences for animal cells. It directly impacts cell volume, nutrient absorption, waste excretion, and cellular communication. Understanding the principles of osmosis and its effects under different osmotic conditions is essential for comprehending physiological processes, diagnosing diseases, and developing effective treatments. The delicate balance of osmotic pressure within and outside cells is crucial for maintaining cellular homeostasis and overall organismal health. Disruptions in this balance can have severe consequences, highlighting the critical role osmosis plays in maintaining the integrity and function of animal life. Continued research into the intricacies of osmosis will undoubtedly yield further insights into the complexity of biological systems and contribute to advances in medicine and biotechnology.
Latest Posts
Latest Posts
-
Lock And Key Model Of Enzyme Action
Mar 19, 2025
-
Integration Of Odd And Even Functions
Mar 19, 2025
-
What Is The Characteristics Of Population
Mar 19, 2025
-
What Years Were Buffalo Nickels Made
Mar 19, 2025
-
Solving Systems Of Linear Equations By Substitution Answer Key
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Does Osmosis Affect Animal Cells . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
