How To Add Formal Charges To Resonance Structures
Muz Play
Mar 19, 2025 · 5 min read
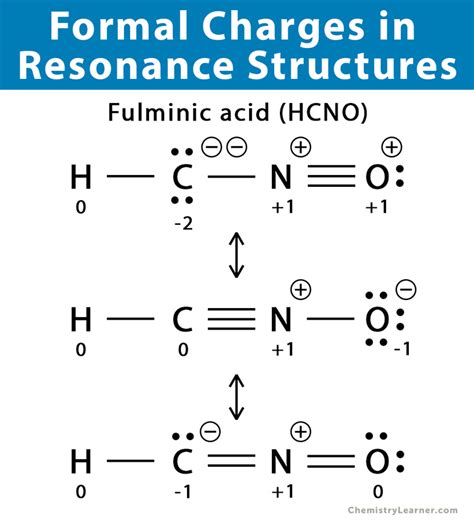
Table of Contents
How to Add Formal Charges to Resonance Structures
Resonance structures are essential tools in chemistry for depicting the delocalized electrons within molecules. They represent different possible distributions of electrons that contribute to the overall structure of a molecule. Accurately assigning formal charges to each atom within these resonance structures is crucial for understanding the molecule's reactivity and properties. This comprehensive guide will walk you through the process step-by-step, ensuring you can confidently add formal charges to any resonance structure.
Understanding Formal Charges
Before diving into adding formal charges to resonance structures, let's solidify our understanding of what formal charges represent. A formal charge is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between the two atoms. It's a bookkeeping tool to help us visualize electron distribution and predict molecular properties, and it does not represent the actual charge on an atom. The sum of all formal charges in a neutral molecule should always equal zero, while the sum in an ion equals the ion's charge.
The formula for calculating formal charge is:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - (1/2 Bonding Electrons)
Where:
- Valence Electrons: The number of electrons in the outermost shell of the atom in its neutral state. This can be found on the periodic table.
- Non-bonding Electrons: The number of electrons that are not involved in covalent bonds (lone pairs).
- Bonding Electrons: The number of electrons involved in covalent bonds.
Step-by-Step Guide to Adding Formal Charges
Let's break down the process of adding formal charges to resonance structures into a series of manageable steps. We'll use examples to illustrate each step.
Step 1: Draw the Lewis Structure (or Resonance Structures)
Begin by drawing the Lewis structure (or all contributing resonance structures) of the molecule or ion. This involves determining the total number of valence electrons, connecting atoms with single bonds, distributing remaining electrons as lone pairs to satisfy the octet rule (or duet rule for hydrogen), and forming multiple bonds if necessary. Remember to consider possible resonance structures where electron pairs can be delocalized.
Example: Let's consider the nitrate ion (NO₃⁻).
First, draw a valid Lewis structure. You should find that multiple valid Lewis structures are possible. This is because the double bond could be on any of the three oxygen atoms. These are resonance structures. Draw all possible structures.
Step 2: Count Valence Electrons for Each Atom
Determine the number of valence electrons for each atom in your Lewis structures. Use the periodic table as a reference. For example:
- Nitrogen (N): 5 valence electrons
- Oxygen (O): 6 valence electrons
Step 3: Count Non-Bonding Electrons for Each Atom
Count the number of non-bonding electrons (lone pairs) around each atom in your Lewis structure. These electrons are not involved in any chemical bonds.
Step 4: Count Bonding Electrons for Each Atom
Count the number of electrons involved in bonds with each atom. Remember that each bond consists of two electrons.
Step 5: Calculate Formal Charge for Each Atom
Now, apply the formal charge formula to each atom in the Lewis structure. Let's calculate the formal charges for the Nitrate ion (NO₃⁻) resonance structures.
Example - Nitrate ion (NO₃⁻):
Let's consider one resonance structure where one oxygen atom has a double bond to the nitrogen atom.
-
Nitrogen (N):
- Valence electrons = 5
- Non-bonding electrons = 0
- Bonding electrons = 8 (4 bonds x 2 electrons/bond)
- Formal charge = 5 - 0 - (8/2) = +1
-
Oxygen with double bond (O=N):
- Valence electrons = 6
- Non-bonding electrons = 4 (2 lone pairs)
- Bonding electrons = 4 (2 bonds x 2 electrons/bond)
- Formal charge = 6 - 4 - (4/2) = 0
-
Oxygen atoms with single bonds (O-N):
- Valence electrons = 6
- Non-bonding electrons = 6 (3 lone pairs)
- Bonding electrons = 2 (1 bond x 2 electrons/bond)
- Formal charge = 6 - 6 - (2/2) = -1
Therefore, in this resonance structure, the nitrogen has a formal charge of +1, one oxygen has a formal charge of 0, and the other two oxygens each have a formal charge of -1. Repeat this process for all other resonance structures of Nitrate ion. You will find that the formal charges will be distributed differently, but the overall charge of the ion will always remain -1.
Step 6: Check the Sum of Formal Charges
Ensure the sum of the formal charges on all atoms equals the overall charge of the molecule or ion. In the case of the nitrate ion, the sum of the formal charges should be -1, regardless of the resonance structure considered. This is a critical check to verify the accuracy of your calculations.
Step 7: Indicate Formal Charges on the Structure
Finally, indicate the formal charge on each atom directly on the Lewis structure by writing the formal charge value (with a '+' or '-' sign) above or below the atom.
Advanced Considerations: Resonance and Formal Charge
-
Minimizing Formal Charges: The most stable resonance structures usually have the minimum number of formal charges. Structures with fewer formal charges are generally more stable.
-
Favorable Formal Charges: If formal charges are present, it's generally more favorable for negative formal charges to reside on more electronegative atoms (like oxygen) and positive formal charges on less electronegative atoms.
-
Resonance Hybrid: Remember that resonance structures are merely contributing representations. The actual molecule is a hybrid of all resonance structures, and the electron density is delocalized across the molecule. Formal charges help us understand the distribution of electron density in this hybrid structure.
-
Dealing with Exceptions: Some molecules might not perfectly obey the octet rule (e.g., molecules with expanded octets). The formal charge calculation still applies; you simply adjust the number of bonding and non-bonding electrons accordingly.
Practice Problems
To master the skill of adding formal charges, practice is essential. Try drawing the Lewis structures and determining the formal charges for the following molecules and ions:
- Carbonate ion (CO₃²⁻)
- Sulfate ion (SO₄²⁻)
- Ozone (O₃)
- Benzene (C₆H₆)
- Acetate ion (CH₃COO⁻)
By working through these examples, and others you can find in textbooks and online resources, you'll build your confidence and expertise in applying formal charges to resonance structures. Remember that understanding formal charges is crucial for predicting reactivity, understanding bonding, and ultimately, gaining a deeper understanding of molecular behavior. Consistent practice will solidify your understanding and make you a more proficient chemist.
Latest Posts
Latest Posts
-
What Is A Node In Physics
Mar 19, 2025
-
What Are Polymers Of Nucleic Acids
Mar 19, 2025
-
What Are The Four Components Of Daltons Atomic Theory
Mar 19, 2025
-
How To Identify Strong And Weak Acids And Bases
Mar 19, 2025
-
What Is The Building Block Monomer Of Nucleic Acids
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How To Add Formal Charges To Resonance Structures . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
